By measuring intracellular proteins with flow cytometry, researchers can better understand cell signaling and functional responses in conditions of health and disease
Flow cytometry is widely used for identifying different cell types based on the expression of surface markers. However, being able to measure intracellular markers by flow cytometry can offer insights into cell signaling and functional responses and reveal how critical processes are dysregulated during conditions of disease. When staining for intracellular proteins, cells must be fixed and permeabilized using carefully optimized protocols to ensure data accuracy. We spoke with Christopher Manning, Associate Director for Flow Cytometry at Cell Signaling Technology, who shared some tips for performing intracellular flow cytometry experiments.
Importance of Intracellular Proteins
Intracellular proteins have essential roles in almost all biological processes, making them potential therapeutic targets for conditions spanning cancer to autoimmune disease. One way in which intracellular proteins exert their effects is through altered expression. For example, under normoxic conditions, the transcription factor hypoxia-inducible factor 1α (HIF-1A) is expressed at very low levels. However, under hypoxic conditions, it is up-regulated to drive the transcription of multiple genes that enable metabolic adaptation to oxygen deprivation. This feature of HIF-1A makes it an important cancer drug target, since it is known to promote tumor metabolism, angiogenesis, and cell survival within the hypoxic tumor microenvironment. “Intracellular proteins may also function by inducing post-translational modifications (PTMs) such as phosphorylation, methylation, or protein cleavage,” reports Manning. “These serve to modulate protein activity, stability, or function, to influence cellular behaviors.”
Intracellular Flow Cytometry Applications
Intracellular flow cytometry can be used for an almost limitless number of applications. “A main use of intracellular flow cytometry is to correlate the cellular phenotype, based on surface marker expression, with cellular function,” explains Manning. “This could include measuring cytokine production, the expression level of transcription factors, or phosphorylation-driven activation of signaling pathways. Intracellular flow cytometry is also seeing increased use for epigenetics, which is the study of how histone and DNA modifications govern gene expression without changing the genetic code.” Other applications of intracellular flow cytometry include its use for studying pluripotency and differentiation when working with stem cells, and its utility for monitoring markers of apoptosis.
Fixation and Permeabilization
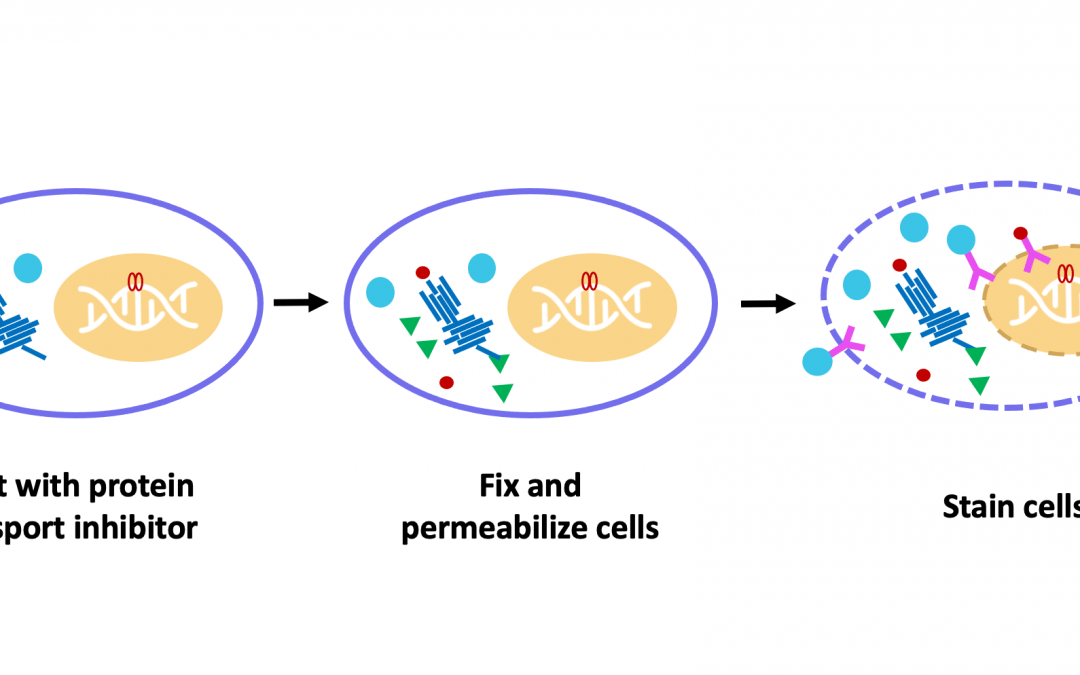
While detecting cell surface markers is relatively straightforward, involving little more than blocking, immunostaining, and washing, detecting intracellular markers requires that several additional protocol steps be performed. Specifically, the cells must be fixed to prevent any changes from occurring during subsequent handling, and permeabilized to allow antibody reagents to access their targets.
“Formaldehyde is commonly used for fixation as it preserves cellular morphology and reduces the loss of soluble proteins,” comments Manning. “Alternatively, samples may be fixed with methanol, which offers the advantage of simultaneously permeabilizing the cells and can thus save time. However, it is important to note that methanol fixation can be disruptive to cellular epitopes, prohibiting antibodies from binding.” When choosing between the different fixation methods, researchers are advised to consult the antibody datasheet for the recommended approach and to perform optimization for their unique model system.
The choice of permeabilization agent can likewise impact antibody specificity and functionality. “Detergents like Triton™ X-100 or saponin are often used for permeabilization,” reports Manning. “Of these, Triton™ X-100 will permeabilize the plasma membrane as well as intracellular membranes, such as those surrounding the nucleus and mitochondria. Saponin is more stringent, permeabilizing only the plasma membrane. Saponin is also reversible, meaning that it should be included in any antibody diluents that are used downstream of permeabilization.”
Tips for Successful Intracellular Flow Cytometry Staining
To ensure that intracellular flow cytometry data are both accurate and reproducible, researchers are advised to plan their experiments carefully and optimize their assay setup using appropriate controls. Here are our top five tips for successful intracellular flow cytometry staining:
-
Understand Your Target
Researching your target of interest is one of the first steps toward generating high quality intracellular flow cytometry data. This includes where in the cell the target is found, how abundant it is, and whether some form of treatment is required to stimulate its expression. “If the protein you wish to detect is mitochondrial or nuclear, you may need to use methanol or Triton™ X-100 for permeabilization,” comments Manning. “But you should always check that this is compatible with other antibodies being used in the same panel, including antibodies for surface markers.”
-
Think About Performing Sequential Staining
If you need to detect a combination of extracellular and intracellular markers in the same experiment, you may wish to consider sequential staining. This involves blocking and staining for surface markers first, prior to fixing, permeabilizing, and staining for intracellular targets. Cell Signaling Technology has developed an Antibody Staining Guide for Flow Cytometry to aid this process, which includes an easy-to-use decision tree for determining an approach that best suits your experimental needs.
-
Select High-Quality Antibody Reagents
When selecting antibodies for your research, look for products that have been proven to detect the target of interest in the selected species and application. “At Cell Signaling Technology, we determine the functionality, specificity, and sensitivity of an antibody in any given assay by adhering to the Hallmarks of Antibody Validation,” says Manning. “The hallmarks comprise six complementary validation strategies that we carefully tailor to each product based on factors including the biological role of the target, the availability of appropriate testing models, and the required assay sensitivity.”
-
Assign Fluorophores Carefully
Because almost any intracellular flow cytometry experiment will necessitate detecting multiple targets, it is important to pay close attention to panel design. Bright fluorophores should be assigned to less abundant markers, and vice versa, and if antibodies will be added prior to methanol permeabilization it is important to avoid using protein fluorophores like phycoerythrin (PE) and allophycocyanin (APC) which will be denatured by methanol. Save those fluorophores for antibodies added after methanol permeabilization is complete.
-
Consider Using Reagents That Are Designed for Intracellular Flow Cytometry
While many of the reagents used for cell surface marker staining are compatible with intracellular flow cytometry, you may wish to consider using products that are optimized for intracellular flow cytometry applications. These include fixable viability dyes such as Ghost Dyes, which can be used in place of common viability stains such as propidium iodide (PI), 7-amino-actinomycin D (7AAD), and 4′,6-diamidino-2-phenylindole (DAPI), all of which are unsuitable for determining cell viability after fixation.
Supporting Your Research
Whether you are detecting intracellular proteins, cell surface markers, or a combination of both, FluoroFinder has developed a suite of tools to help you with panel design and your research. FluoroFinder’s Panel Builder can walk you through the fluorophore selection process to ensure you’re using reagents optimal for your specific cytometer configuration and experimental biology. Our Panel Builder‘s antigen density feature can help you find fluorophores optimal for your target’s antigen expression level to help you avoid poor signals and high background noise. If you’re looking for a specific reagent, our Dye Directory is a useful resource to learn more about the spectral properties and applications of over 1,100 dyes and help you find products from all major suppliers.
Cell Signaling Technology also offers a comprehensive array of open-access materials, including a Flow Cytometry Resource Center with protocols, a troubleshooting guide, and further tips for success.
Sign-up for our eNewsletter to receive regular updates about ICC, IHC, and other fluorescence-based techniques, and be among the first to hear about the latest products