Selecting the right secondary antibodies, and optimizing them for use, is essential for reliable immunoassay data.
When developing an immunoassay, researchers typically pay close attention to primary antibodies, carefully scrutinizing the product datasheets for proof of target specificity and functionality in a particular assay. Yet, all of that hard work can be undone in moments by failing to use the right secondary antibody for detection. If you want your immunoassay data to make sense, secondary antibody selection should be considered equally as important as choosing a suitable primary antibody. We sat down with Miranda Lewis, Ph.D., Marketing Manager at Jackson ImmunoResearch, to discuss challenges with secondary detection and ways of avoiding common mistakes.
What is Secondary Detection?
Immunoassays can be broadly divided into two categories, direct and indirect, based on the types of antibodies that are used for detection. Direct immunoassays use labeled primary antibodies, offering the advantages of fewer protocol steps and less chance of non-specific signal compared with indirect detection. However, limitations of direct detection include the potential for labeling to compromise antibody binding to the target protein and the higher price tag that often comes with labeled primaries.
Indirect immunoassays instead combine unlabeled primary antibodies with labeled secondary antibodies for detection. “A major advantage of indirect detection is that it offers access to a wider range of reporter molecules,” says Lewis. “This makes it easier to switch between different labels, which can be especially helpful when developing multiplex immunofluorescence experiments. In addition, indirect detection can provide signal amplification due to multiple secondaries binding to each primary antibody, increasing researchers’ chances of detecting less abundant targets.”
On the flipside, drawbacks of indirect detection are that it extends the experimental workflow with the extra incubation and wash steps, and has a higher associated risk of non-specific binding. Yet, the latter can be avoided by selecting the right secondary antibody reagents and being thorough with experimental design.
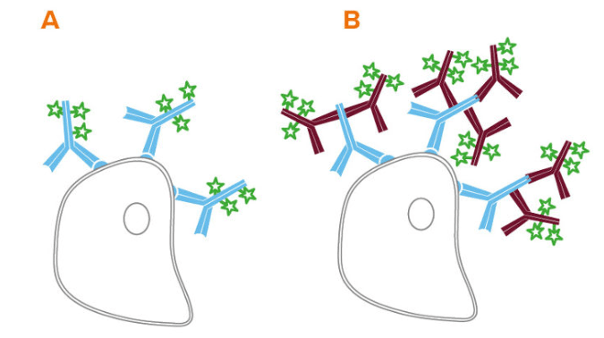
Figure 1. Direct (A) and indirect (B) flow cytometry
Applications of Secondary Detection
Secondary antibodies are supplied as a broad range of formats, giving them utility for many different research applications. Common label types include enzymes such as horseradish peroxidase (HRP) and alkaline phosphatase (AP), which are typically paired with either chromogenic or luminescent substrates for techniques including western blotting, immunohistochemistry (IHC), and ELISA. Secondary antibodies are also available labeled with fluorophores such as R-phycoerythrin (RPE), fluorescein isothiocyanate (FITC), and the Alexa Fluor® dyes, which are widely used for multiplexed flow cytometry, immunocytochemistry (ICC), and various bead-based immunoassays. Additionally, secondary antibodies may be labeled with biotin, enabling detection with a streptavidin-enzyme or streptavidin-fluorophore conjugate, or be supplied bound to agarose or magnetic beads for use in pulldown assays.
Addressing Secondary Detection Challenges
Challenges for secondary detection center mainly on preventing non-specific antibody binding that can manifest as false positive results or high background signal. Ways of addressing these problems include the following:
-
Select the Right Secondary Antibodies
When an immunoassay is designed to measure just a single analyte, secondary antibody selection is relatively straightforward. For example, if the primary antibody was produced in a mouse host, an anti-mouse secondary antibody is required for detection. But, when performing multiplexed experiments with secondary detection, there are several other factors to consider.
“If multiple secondary antibodies are combined in the same experiment, we recommend that they share the same host species, if possible, to stop them from binding to one another,” cautions Lewis. “Researchers should also choose secondary antibodies that have been cross-adsorbed against the tissue species, and any other primary antibodies being used in the same experiment, to prevent background from cross-reactivity.”
An easy way of increasing panel size when performing indirect detection is to use class- or subclass-specific secondary antibodies. “Mice typically encode for IgG1, IgG2b, and IgG3, and will express either IgG2a or IgG2c depending on their strain, which allows for combining multiple anti-mouse IgG subclass-specific secondary antibodies,” explains Lewis. Similarly, rats express IgG1, IgG2a, IgG2b, and IgG2c, whereas rabbits have only one IgG subclass.
-
Choose an Appropriate Blocking Agent
Blocking is an essential protocol step that serves to prevent non-specific antibody binding. When performing indirect detection, it is advised to use normal serum from the same host species as the secondary antibody for blocking since this contains antibodies that will attach to any reactive sites, prohibiting secondary antibody binding later on.
Alternatively, bovine serum albumin (BSA) may be used for blocking, provided it is free of contaminants that could interfere with immunodetection. “Most commercial preparations of BSA contain contaminating bovine IgG that may become an antigen for cross-reacting secondary antibodies,” reports Lewis. “Using IgG-free, protease-free BSA is advised to avoid reduced antibody activity and/or increased background.”
-
Don’t Overlook the Impact of Endogenous Proteins
As well as performing general blocking, it can often be necessary to introduce extra blocking steps into the experimental workflow. This is especially the case for IHC and ICC, when blocking endogenous immunoglobulins, enzymes, and/or biotin is frequently required to prevent them from interfering with the assay readout.
“If indirect detection will involve using a primary antibody from the same host species as the sample, researchers are advised to block endogenous immunoglobulins with an unconjugated Fab fragment antibody immediately after routine blocking,” comments Lewis. “For example, a mouse sample could be incubated with a goat anti-mouse Fab fragment, which would bind to the endogenous IgG and allow for subsequent incubation with a mouse primary followed by an anti-mouse secondary antibody.”
Blocking endogenous peroxidase or phosphatase activity should be considered when HRP- or AP-conjugated antibodies are respectively used for detection. Endogenous HRP activity can be blocked by incubating samples in a dilute solution of hydrogen peroxide before adding the primary antibody, while blocking endogenous AP can be accomplished with levamisole, or with a 1% solution of acetic acid when working with intestinal tissues.
Blocking endogenous biotin is advised if using a biotin-based detection system such as the avidin-biotin-complex (ABC) method. This can be done by incubating the sample with avidin followed by biotin (to block any open sites on the avidin molecule) prior to adding the primary antibody.
-
Include Controls to Confirm Secondary Antibody Specificity
Once you have identified a suitable secondary antibody, it is recommended to confirm its specificity for the intended primary antibody target, particularly if the antibody pair will be included in a multiplexed panel. Secondary antibody specificity can easily be checked by attempting to label the ‘wrong’ primary antibodies, and it is also advised to run ‘secondary antibody only’ controls (omitting the primary antibody incubation step from the experimental workflow) to check for any sources of non-specific secondary antibody binding. Lewis also recommends testing for sample autofluorescence in the channels being used prior to staining. “Using the red and far red channels for detection where possible, rather than the UV and green channels, will help to avoid inherent signal resulting from autofluorescence,” she says.
-
Consider Using Secondary Antibody Alternatives
For researchers wishing to reduce the number of protocol steps involved in their immunoassay, but for whom direct detection is not an option, Jackson ImmunoResearch offers an alternative. “FabuLight™ antibodies are Fab fragment secondary antibodies specific to the Fc region of IgG or IgM primary antibodies,” reports Lewis. “They are available conjugated with 9 different fluorophores and biotin, and allow for labeling of primary antibodies prior to incubation with cells or tissue.” While using FabuLight antibodies saves time compared with sequential staining protocols, researchers should note that because FabuLights are not cross-adsorbed against other species, it may be necessary to block endogenous immunoglobulins (as previously described).
Supporting Your Research
Whether you’re performing a single analyte western blot or a complex multiparameter flow cytometry experiment, FluoroFinder has the tools you need to streamline experimental design. Use our Antibody Search tool to quickly identify antibodies for your chosen target, species, and application – we’ve got you covered for both direct and indirect detection. And try out our Spectra Viewer for microscopy to visualize your fluorophores in the context of your laser and filter configuration