Multiparametric flow cytometry allows researchers to detect different cell types within a heterogeneous population. When combined with cell sorting and sequencing, it can reveal how novel cell subsets function in conditions of health or disease. A major challenge for those performing flow cytometry-based research lies in distinguishing cell-cell complexes (doublets) from singlet cells. Not only is this critical for correct interpretation of data, but it also underpins the purity of sorted cell populations. Here, we provide a quick recap of flow cytometry principles to explain how doublets occur, before commenting on how they can be gated out during analysis. We also share sample preparation tips for ensuring a single-cell suspension and highlight a recent publication discussing the use of molecular signatures for doublet discrimination.
Flow cytometry principles and the occurrence of doublets
Flow cytometers function by using a stream of fluid to direct cells in single file past an interrogation point, where one or more lasers are focused. As the cells cross the interrogation point, they scatter the laser light and, at the same time, any labeled antibodies that are bound to cellular markers fluoresce. The resultant signals are guided toward specialized detectors known as photomultiplier tubes (PMTs), each measuring light of only a specific wavelength. When light hits a PMT, it causes it to emit a voltage pulse known as an event; by gathering data from thousands of different events, the flow cytometer provides a graphical interpretation of the sample.
Each event is characterized by its height (H) and width (W) – equating to the PMT output and the time taken for the cell to pass through the interrogation point, respectively; it also has an area (A), which is a property of both H and W. Critically, for something to be considered an event, not only must the signal generated by the PMT be greater than the threshold set by the end user to reduce unwanted background signal, but it must also return to baseline levels. Where two cells pass through the interrogation point close together, the voltage pulse may not drop back down to the baseline between them, and it is this that can result in their mistaken classification as a single, large event.
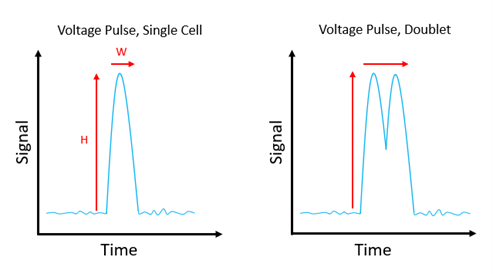
Figure 1: Voltage pulse for a single cell, versus voltage pulse for a doublet.
Doublet discrimination
Accurately identifying and excluding doublets is essential for correct interpretation of flow cytometry data. For those performing cell cycle analysis by measuring cellular DNA content, doublet discrimination is easily achieved using the DNA dye channel; for everyone else, forward scatter (FSC) and side scatter (SSC) are the best options since they are linearly scaled. While various parameter combinations can be applied, largely based on personal preference, those using FSC are the most common. Once doublets have been excluded, all subsequent analyses can be gated on the remaining region.
| x-axis | y-axis |
| FSC-A | FSC-H |
| FSC-A | FSC-W |
| FSC-H | FSC-W |
Common doublet discrimination strategies.
Sample preparation tips for ensuring a single-cell suspension
Doublets are an unavoidable fact of flow cytometry, tending to increase in number with higher sample densities and faster flow rates. Although they can be gated out, there are several other steps researchers can take to limit their occurrence as well as avoid the presence of larger clumps that could block the flow cytometer. Best practice recommendations include handling cells gently to prevent the release of DNA, which can bind cells together, and adding DNase to samples to minimize any such effects; avoiding harsh centrifugations that could cause cellular damage or lead to over-pelleting; and using resuspension buffers free of Ca2+/Mg2+ ions and containing a low concentration (<2%) of protein. It is also advised that samples be filtered through an appropriately-sized cell strainer before being introduced into the flow cytometer.
Using molecular signatures for doublet discrimination
A recent publication from the La Jolla Institute for Immunology describes the development of a novel strategy for distinguishing cell-cell complexes from singlet cells using robust molecular signatures. Having noticed that doublets comprising T cells bound to other immune cells (e.g., monocytes or B cells) are often present in human peripheral blood samples acquired by conventional flow cytometry, the authors chose to investigate whether select cellular markers could be used for doublet discrimination. Included among their findings was the observation that marker expression levels differed between singlet cells and the corresponding doublets, prompting further interrogation of ‘dual-expressing’ cells by imaging flow cytometry and direct microscopy imaging. When this strategy was applied to a previously published dataset describing a cell population with mixed T cell and B cell lineage properties, it suggested that the new cells were likely to be T cell–B cell complexes.
Supporting flow cytometry-based research
Whatever the goal of your flow cytometry experiment, FluoroFinder offers a suite of solutions to simplify reagent selection and panel design. Use our Antibody Search function to find antibodies that have been validated for flow, or take a look at our Spectra Viewer to quickly compare over 1,000 fluorophores from all suppliers in one intuitive platform. For panel design, our Panel Builder lets you view the fluorophore and antibody offerings of >60 suppliers in a single resource. And of course, if you need additional guidance, our technical support team is always available to help.
Sign up for our eNewsletter to receive regular updates about flow cytometry-based research, including the very latest antibody and fluorophore offerings.




