The use of mass cytometry is growing and includes its adoption for clinical applications
Mass cytometry is a variation of flow cytometry that uses antibodies labeled with metal tags rather than fluorophores. Also known as CyTOF® flow cytometry, in deference to Standard BioTools’ widely used technology platform, mass cytometry circumvents the need to address spectral overlap and autofluorescence that presents challenges for fluorescence cytometry experiments. To learn more about mass cytometry, we spoke with Jennifer Ellis, Principal Scientific Writer at Standard BioTools™, who explained the basic principles and advantages of the technique, as well as offered some tips for panel design.
Basic principles of mass cytometry
During a typical mass cytometry experiment, a single-cell suspension is stained for various protein targets using antibodies labeled with different heavy metal isotopes. The sample is then introduced into the CyTOF and injected into an inductively coupled plasma for ionization. Following this, the ions are quantified based on their time-of-flight (metal tags have a longer time-of-flight due to their higher mass-to-charge ratio) and the signals corresponding to each tag are correlated back to the respective marker. The mass cytometry workflow is shown in Figure 1. “Because CyTOF detects mass, it is highly precise,” reports Ellis. “And, with the potential for over 100 distinguishable tags, CyTOF is seeing utility for applications spanning basic research through to clinical research and monitoring of disease treatment.”
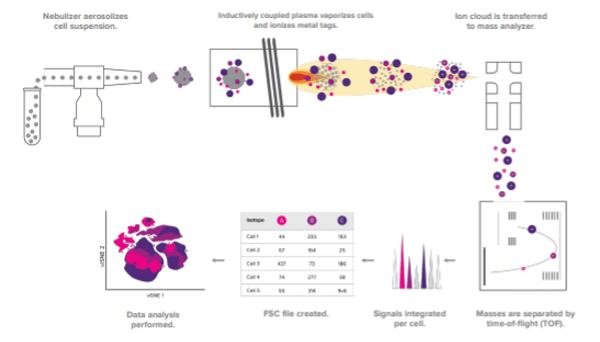
Figure 1. The CyTOF workflow.
Differences between mass cytometry and fluorescence cytometry
Besides the types of labels that are used, another key difference between mass cytometry and fluorescence cytometry lies in the complexity of the signals that are produced. “While CyTOF produces distinct signals, fluorescence yields muddled signaling that needs compensation and unmixing with the aid of multiple controls even for small panels,” notes Ellis. “A consequence of this is that CyTOF allows for more markers to be detected per run, including better detection of intracellular proteins, which can increase researchers’ chances of capturing rare or unexpected cell populations within limited sample material.” Specifically, mass cytometry allows for the detection of 50 or more markers, whereas most fluorescence cytometry experiments are designed to measure around 5-10 markers. However, a limitation of mass cytometry is that it prohibits the cells from being used in downstream applications. A comparison of the different signal outputs for fluorescence cytometry and CyTOF is shown in Figure 2.
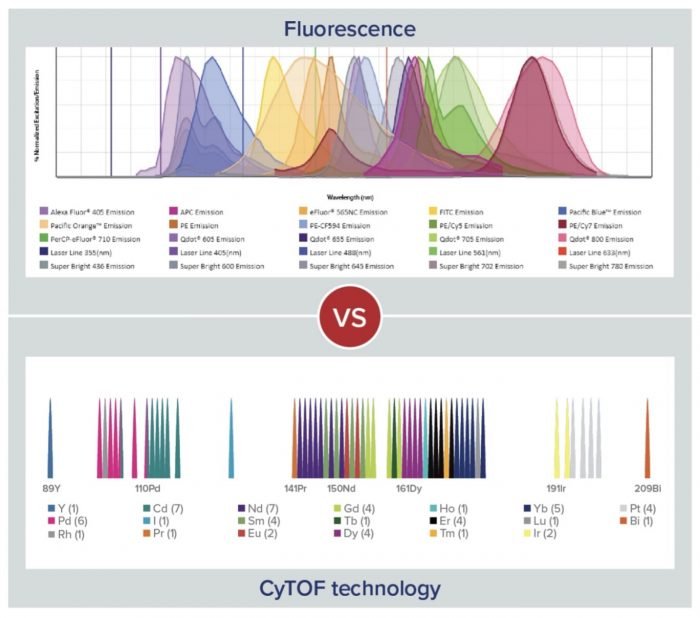
Figure 2. Comparison of the different signal outputs for fluorescence cytometry and CyTOF.
Advantages of mass cytometry
A major advantage of mass cytometry is that the data quality is outstanding. This is largely due to the discrete signaling produced by metal tags, as well as the fact that mass cytometry is not complicated by the need to address spectral overlap and autofluorescence. “Mass cytometry also benefits from easy panel design with the ability to swap in markers as required, which is difficult with fluorescence, as well as offers barcoding capabilities for multiplexing to both minimize technical variability and save sample,” notes Ellis. “Building larger panels enables the use of more functional and intracellular markers, which can provide deeper insights into sample material. Now, it is even possible for the mass cytometry workflow to be automated using CyTOF XT™, which enables up to 13 sample tubes to be acquired unattended.” Another important advantage of mass cytometry is that it allows researchers to freeze their samples after staining as the metals are very stable and the signal doesn’t fade, which makes it great for longitudinal or multi-site studies.
Considerations for panel design
Although panel design is more straightforward for mass cytometry than fluorescence cytometry, it is still important to follow best practices. These include optimizing the method used for sample collection and selecting well-validated antibody reagents. “For immune profiling, the Maxpar® Direct™ Immune Profiling Assay™ provides researchers with a strong starting point,” says Ellis. “This is a dry-format 30-marker antibody panel that is preoptimized for fresh whole blood and PBMCs. It can be used in combination with the automated Maxpar Pathsetter™ software to do automated cell analysis of 37 immune cell populations.”
The Maxpar Direct Immune Profiling Assay can be extended with pre-configured Maxpar Direct Expansion Panels, which fit into the open channels to support specific applications. “Using the Maxpar Direct Expansion Panels, researchers can investigate the cell cycle, monitor cell signaling pathways, or detect phenotypic and functional markers associated with specific cell types including stem cells, T and B cells or monocytes/macrophages,” reports Ellis. “It is also possible to simultaneously detect other markers of interest by adding in individual Maxpar antibodies.” Figure 3 shows the Maxpar Direct Immune Profiling Assay in combination with the Maxpar Direct NK Cell Expansion Panel.
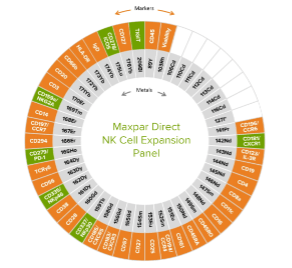
Figure 3. Cellular markers detected by the Maxpar Direct Immune Profiling Assay (orange) and the Maxpar Direct NK Cell Expansion Panel (green).
Additional Resources
Standard BioTools has developed a wide array of resources to help researchers maximize the value of their mass cytometry experiments. These include application notes for designing a 47-marker immune phenotyping panel, cryopreserving stained samples, and performing live-cell barcoding, as well as blogs on the advantages of CyTOF® technology and what high-parameter cytometry means for scientific research.




