Functional probes are essential tools for flow cytometry, with utility for studying a wide array of cellular processes. Here, we speak with Alexis Madrid, Ph.D., Assistant Director of Bioscience at Biotium and Chris Manning, Associate Director, Flow Cytometry at Cell Signaling technology (CST®) to learn about some of the functional probes available to researchers.
What are functional probes?
In general terms, functional probes are chemicals that serve as detection agents based on how they interact with their target molecules in a complex biological environment. “Functional probes emit a signal that reflects a biological state of the cell, as opposed to simply binding to a specific molecular target,” explains Madrid. “These probes are very useful in flow cytometry because they can provide information about cellular processes such as apoptosis, necrosis, proliferation, and mitochondrial function.”
But why would you choose to use a functional probe for flow cytometry, a technique that goes hand in hand with antibody-based detection? “While conjugated antibodies are widely utilized to measure intracellular protein levels and post-translational modifications in flow cytometry assays, they can only bind those targets when cells are fixed and permeabilized,” explains Manning. “When an assay requires live cells, membrane-permeant fluorescent probes are an ideal solution. They are able to enter live cells and either label a target of interest or be acted on by specific enzymes to report their activity through a change in fluorescence.”
Types of functional probes
Despite not offering the diversity of readouts available with antibodies, functional probes allow for studying many different cellular states. The following are some of the main types of functional probes that are used for flow cytometry.
-
Cell Viability Probes
Cell viability is a term that describes the proportion of live, healthy cells within a population. It is commonly assessed using DNA intercalating agents such as propidium iodide (PI), 7-aminoactinomycin D (7-AAD), and 4′,6-diamidino-2-phenylindole (DAPI), which can enter only dead cells that have leaky membranes. Another popular approach is to use Calcein-AM, a cell-permeant dye that is cleaved by intracellular esterases to produce green fluorescence. However, because these dyes are largely incompatible with fixation (many commonly used DNA binding viability dyes cannot be fixed after staining and Calcein-AM cannot be hydrolyzed by fixed cells), various alternatives have been developed.
“Biotium’s Live-or-Dye™ Fixable Viability Stains are cell membrane impermeant amine-reactive dyes,” reports Madrid. “They enter dead cells that have compromised membrane integrity, where they covalently label free amines on intracellular proteins.” Live-or-Dye™ labeling permits fixation and permeabilization with no loss of fluorescence or dye transfer between cells and offers a choice of 14 different colors for flexible panel design. Ghost Dye™ Fixable Viability Dyes, available from CST, are based on similar principles to Live-or-Dye™ labeling, allowing for non-viable cells to be easily excluded from flow cytometry analysis.
-
Cell Proliferation Markers
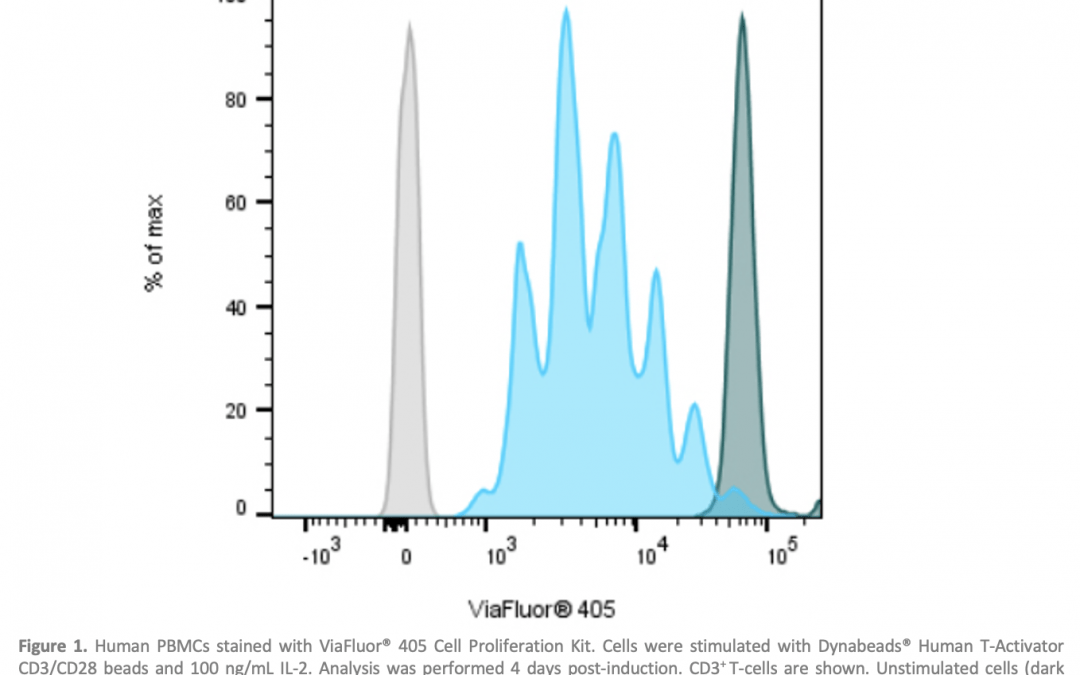
Cell proliferation refers to the increase in cell number resulting from cell division. One of the best-known ways of measuring cell proliferation by flow cytometry is to detect the incorporation of a nucleoside labeling agent, such as 5-bromo-2′-deoxyuridine (BrdU) or 5-ethynyl-2’-deoxyuridine (EdU), into newly synthesized DNA using a fluorescently-labeled antibody. In addition, methods involving covalent labeling of intracellular proteins by fluorescent dyes are common. Proliferation is measured by the signal reducing by half after each cell division. Biotium’s ViaFluor® SE Cell Proliferation Kits and CST’s Cell Proliferation Tracer Kits work on this basis, whereby each cell division that occurs after labeling produces a dimmer fluorescent peak on a flow cytometry histogram (Figure 1).
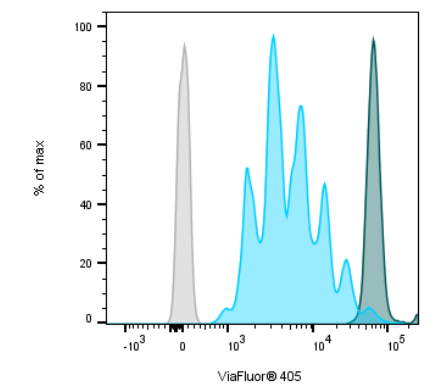
Figure 1. Human PBMCs stained with ViaFluor® 405 Cell Proliferation Kit. Cells were stimulated with Dynabeads® Human T-Activator CD3/CD28 beads and 100 ng/mL IL-2. Analysis was performed 4 days post-induction. CD3+ T-cells are shown. Unstimulated cells (dark peaks) and unstained cells (gray) are included for comparison. Image provided by Biotium.
-
Apoptosis Detection Probes
Apoptosis is a form of programmed cell death that is required for normal growth and development, as well as for removing potentially harmful cells, including those that are virus-infected or DNA-damaged. It is characterized by cell shrinkage, membrane blebbing, nuclear condensation, and DNA fragmentation, and is predominantly regulated by caspase enzymes. Functional probes for apoptosis target many of these characteristics.
“Apoptosis is frequently detected in cells and tissues using antibodies that bind to caspase and PARP proteins only when those proteins are cleaved during the apoptosis signaling cascade,” says Manning. “In live cell assays there are several well-characterized functional probes that work as robust indicators of apoptosis. These probes enable researchers to accurately quantify apoptotic events when fixation to halt biological processes is not an option.”
TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) is an established method for monitoring apoptosis. It involves the enzymatic linkage of a fluorophore-conjugated nucleotide to the 3′ end of fragmented DNA, whereby apoptosis is associated with an increase in TUNEL staining (Figure 2).
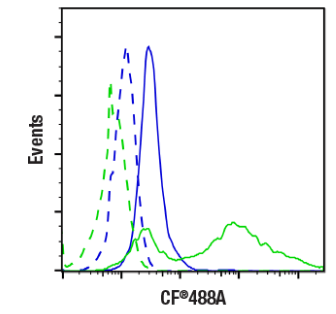
Figure 2. Flow cytometric analysis of Jurkat cells, untreated (blue) or treated with Staurosporine (1 μM, 3 hours, green), unlabeled (dashed lines) or labeled with TUNEL Assay Kit (Fluorescence, 488 nm) (solid lines). Image provided by CST.
Another well-known approach involves using fluorescently-labeled Annexin V to identify phosphatidylserine (PS) at the cell surface. In healthy cells, PS is expressed at the cytosolic side of the plasma membrane. However, during apoptosis, it translocates to the extracellular side of the membrane, leaving the cell membrane intact. Often, researchers will combine Annexin V staining with staining for PI; cells that are positive for both markers demonstrate later stage apoptosis and early necrosis.
Madrid highlights Biotium’s NucView® Caspase-3 Substrates, novel fluorogenic DNA dyes that are coupled to the caspase-3/7 recognition sequence (DEVD). “When introduced to cells, NucView® Caspase-3 Substrates are initially non-fluorescent and able to penetrate the plasma membrane,” she says. “During apoptosis, cleavage by caspase-3 or caspase-7 releases the DNA binding dye and leads to nuclear fluorescent staining. NucView® Caspase-3 Substrates offer a significant advantage over Annexin V probes because they are able to monitor apoptosis in real-time.”
-
Cellular Stress Indicators
Various types of cellular stress stimuli are known to trigger apoptosis. Examples include intracellular iron, lipid peroxidation, and decreased mitochondrial membrane potential. For molecular iron sensing, CST offers FerroOrange, which demonstrates increased fluorescence at 580 nm upon binding to free iron ions in living cells. Lipid peroxidation can be detected with BODIPY 581/591 C11, a fluorescent dye that localizes to membranes and undergoes a shift in fluorescent emission from red to green upon oxidation of its fatty acid analog. To measure mitochondrial membrane potential, researchers can use JC-1, a membrane-permeant cyanine dye that localizes to mitochondria and fluoresces red in healthy cells, and localizes to the cytoplasm and fluoresces green in apoptotic cells with depolarized mitochondrial potential.
Biotium’s potential-dependent mitochondrial stains include MitoView™ 633 and Aquaphile™ JC-1, both of which may be used to asses cellular stress in living cells. “MitoView™ 633 is a far-red dye that becomes brightly fluorescent upon accumulation in the mitochondrial membrane,” says Madrid. “It shares the same optimal emission as Cy®5 and other far-red dyes, but also has visible red fluorescence that allows for detection with Cy®3 settings. Aquaphile™ JC-1 is formulated differently from the original ratiometric JC-1 dye. This new version offers improved solubility and signal-to-noise without alterations to the chemical structure.”
-
Intracellular Signaling Pathway Probes
Intracellular signaling pathways are usually interrogated using antibodies. CST specializes in producing antibodies for these types of readouts and has developed a comprehensive array of Interactive Pathways to help guide product selection. Intracellular messengers, such as calcium (Ca2+), may also be used for studying signaling pathways. The most widely used method for Ca2+ detection employs fluorescent Ca2+ indicators, as pioneered by Roger Tsien in the late 1980s (1). Biotium offers a variety of calcium indicators that differ in terms of their Ca2+ dissociation constants (Kd) or Ca2+ response range, excitation/emission wavelengths, spectral shift, and relative fluorescent quantum yields.
Supporting your research
A growing number of functional probes is available for flow cytometry, many of which can be combined with other readouts for deeper interrogation of your samples. Streamline your research by using FluoroFinder’s Fluorescent Dye Directory to find functional probes that are compatible with your flow cytometer, then simplify panel design with our Spectra Viewer, which lets you view and compare the spectral properties of more than 1,000 dyes alongside instrument-specific laser and filter configurations.
https://www.sciencedirect.com/science/article/abs/pii/S0091679X08609784