Fluorescence recovery after photobleaching is a technique for characterizing the mobility of cellular molecules
Fluorescence recovery after photobleaching (FRAP), also known as fluorescence redistribution after photobleaching, is a microscopy-based technique for tracking the movement of fluorescent molecules over time. When FRAP was first described in 1976, Axelrod et al. primarily focused on monitoring the lateral transport of lipids in the cell membrane. However, other applications of FRAP have since been identified, including its use for studying the dynamic behavior of proteins inside cells. To conduct a successful FRAP experiment, it is crucial to select fluorophores that can withstand image acquisition without photobleaching. FluoroFinder’s Dye Directory can aid in this selection process.
Principles of FRAP
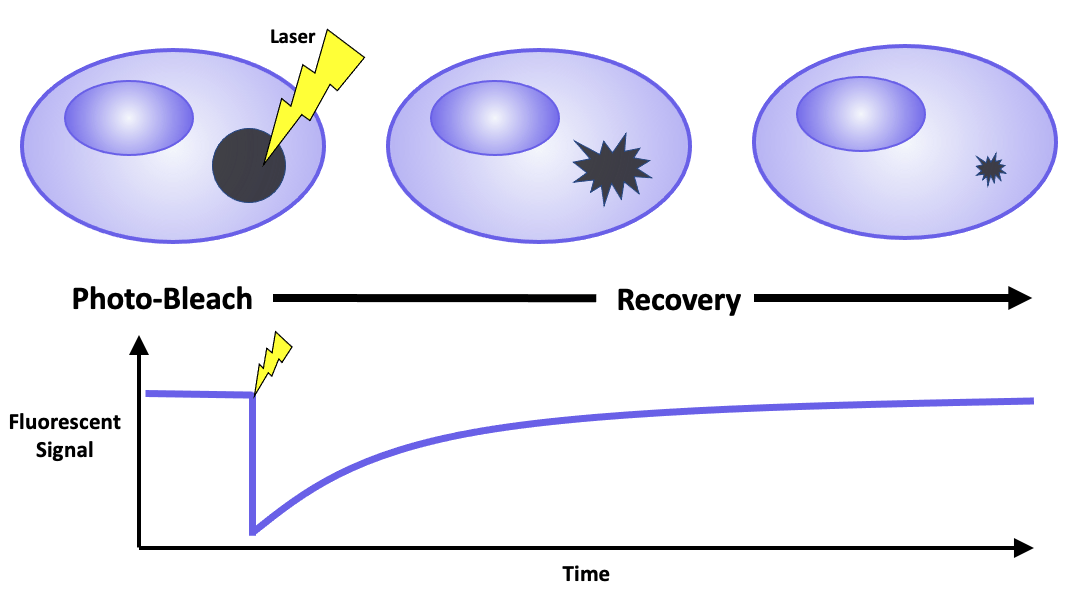
FRAP requires fluorescently labeling a target of interest by either expressing it as a fusion protein or tagging it with reactive ligands that bind to fluorescent dyes. This labeled region is then observed using a laser scanning confocal microscope. The visualization process comprises of three steps. First, a low-intensity laser beam images the cells and identifies a region of interest. A high-intensity laser beam then photobleaches the designated area, leading to photon-induced chemical damage and covalent modification that impairs the fluorophores’ ability to fluoresce. FInally, a low-intensity laser beam is used to track fluorescence redistribution. Measured parameters include percent recovery, the relative amount of fluorescence that returns to the photobleached region relative to the initial intensity, and the regeneration time required to recover half of the original fluorescence. In addition, the ratio between the mobile and immobile fluorescent molecules can be calculated to infer the properties of the sample, such as its viscosity.
FRAP Applications
Although FRAP was initially used to monitor the lateral diffusion of proteins in cell membranes, this technique is now used to analyze other membrane-associated proteins involved in cell adhesion, migration, endocytosis, and signal transduction. Supported lipid bilayers are becoming a more commonly used system for this technique. These minimalistic model systems simulate the functionality of native cell membranes and are more compatible with many common analytical platforms. In addition, FRAP is gaining popularity as a technique for investigating protein dynamics within cells. Its intracellular applications include determining the mobility pattern of known ligand-activated transcription factors by integrating FRAP with single-molecule microscopy, as well as measuring organelle connectivity and membrane trafficking pathways through various modifications of the original method.
Variations of FRAP
Several advanced techniques have been developed to expand the scope of FRAP. Fluorescence loss in photobleaching (FLIP) examines the interconnectivity of different cellular organelles by repeatedly photobleaching the same region of interest. Inverse FRAP (iFRAP) tracks the movement of fluorescent proteins out of intracellular components by photobleaching the area surrounding the region of interest.
Fluorescence localization after photobleaching (FLAP) is a useful approach for analyzing protein mobility and dynamics that occur within a short time frame. This technique utilizes two distinct fluorophores, the target photobleached fluorophore and an unbleached reference fluorophore colocalized with the target. The fluorescence intensities of both fluorophores are measured to generate ratiometric measurements that serve as direct indicators of protein movement.
How to Minimize Photobleaching
Although photobleaching is critical in the FRAP process, it should be avoided during imaging. Fading of the fluorophore during the experiment can lead to misleading results. We recommend the following practices to minimize photobleaching:
- Selecting fluorophores resistant to photobleaching – Green Fluorescent Protein (GFP) and its derivatives are often used as fusion proteins, and the Alexa Fluor® and DyLight™ dyes are also popular for FRAP.
- Reducing bright light – Choosing a lower intensity light source, using neutral-density filters, or decreasing the gain settings on the microscope can minimize bright light.
- Limiting sample exposure – Focusing on the region of interest using transmitted light, focusing on an area adjacent to the target region, or reducing exposure time can limit sample exposure.
- Using anti-fade mounting media – Refer to the manufacturer’s instructions to identify a product that is compatible with your chosen fluorophore.
Supporting FRAP-Based Research
FluoroFinder offers a range of intuitive tools to help design and execute your FRAP experiments. Browse our multi-supplier Fluorescent Dye Database to find fluorophores compatible with your microscope’s configurations that are resistant to photobleaching. Visualize your selected fluorophores within the context of your microscope with our Spectra Viewer for Microscopy and find conjugated antibodies validated for your application.