Biological Controls
Biological controls comprise positive and negative samples, which are respectively cell types that are known to express or lack the target of interest. Examples of positive controls include characterized cell lines, cells which have been transfected to guarantee positive staining, and cells that have undergone some form of treatment to stimulate marker expression. Negative controls also include characterized cell lines, as well as cells in which marker expression has been knocked out using CRISPR or RNAi. For help with identifying relevant biological controls, researchers can use open access resources such as PAXdb and The Human Protein Atlas, refer to antibody datasheets, and review published data.
Reagent Controls
Reagent controls serve to ensure that flow cytometry reagents – especially antibodies and fluorophores – are working as expected, and being used at the correct concentrations. Included among these, antibody titration is essential to avoid using too much antibody (which can lead to non-specific binding) or too little antibody (which can reduce assay sensitivity). Isoclonal controls are also important to confirm that the target cells are not binding to fluorophore labels. They involve staining the sample with each antibody conjugate in the presence of excess unlabeled antibody before measuring the resultant fluorescence; the absence of a fluorescent signal rules out non-specific fluorophore binding. Additionally, lot-to-lot comparisons should be performed every time a batch of reagent is changed.
Viability Controls
Viability controls allow dead and dying cells to be gated out during flow cytometry analysis, thereby mitigating the unwanted effects of their increased autofluorescence and non-specific antibody binding. Although dead cells and cellular debris can be removed from flow cytometry analyses by generating plots of forward scatter versus side scatter, using viability dyes is widely recognized to provide more accurate results. Common viability dyes include 4′,6-diamidino-2-phenylindole (DAPI), a blue nuclear stain which is excluded from live cells by the intact plasma membrane, and 7-amino actinomycin D (7-AAD), which functions in a similar manner but fluoresces red upon DNA binding. Other examples include acridine orange/propidium iodide, which stains live cells green and dead cells red, and various fluorescent conjugates of Annexin V, which binds the apoptosis marker phosphatidylserine and is often combined with a nuclear stain such as propidium iodide.
Unstained Controls
Unstained controls allow researchers to determine the level of background fluorescence (including cellular autofluorescence) in samples and are used for setting voltages and negative gates. They are produced by processing samples as normal, but without adding antibody conjugates, which ensures that any differences between the stained and unstained samples are not due to how the cells have been handled.
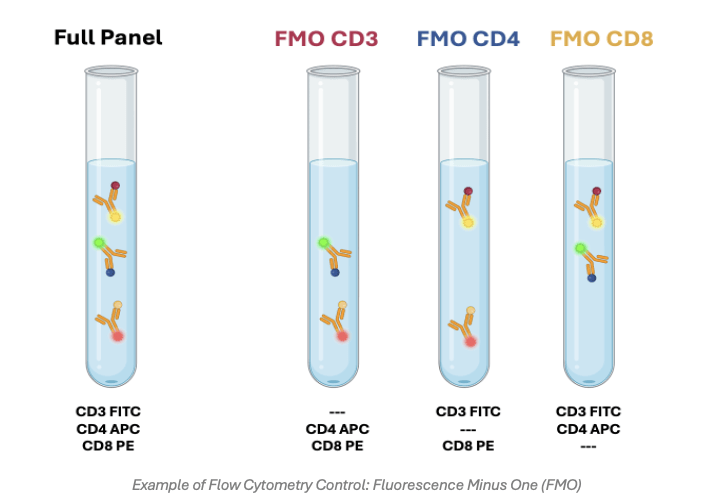
Fluorescence Minus One (FMO) Controls
Fluorescence minus one controls provide a means of addressing spectral overlap between different fluorophores. They consist of samples that have been stained with all but one of the fluorophores in a multicolor flow cytometry panel, whereby the contribution of those fluorophores to the detection channel of interest is then measured. FMO controls are crucial for setting gates, especially when detecting rare events or emerging antigens such as cell activation markers.
Single Color Compensation Controls
Compensation controls are used for determining the spread of a fluorophore into channels beyond that being used for its detection. They typically consist of single fluorophore stained cells, which are often preferred if the target of interest is highly expressed and large amounts of the sample are available, or antibody capture beads, which are better suited to low abundance targets. When preparing compensation controls, the fluorophores should exactly match those being used in the flow cytometry experiment (right down to the lot number), and the measured fluorescence should be at least as bright as that of the sample. Also, the compensation algorithm should be performed with both a positive and a negative population.
Isotype Controls
Isotype controls are antibodies that share the same isotype as a target-specific antibody in terms of species, heavy and light chain, fluorophore, and fluorophore: antibody ratio, but which are raised against a surface antigen which is lacking from the cell type being studied. Although isotype controls were once the go-to negative control for flow cytometry experiments, being widely used to investigate non-specific antibody binding, they have fallen from favor in recent years for introducing yet another experimental variable.
Streamline Experimental Design and Execution
Whether you’re planning to detect just a single cell type, or designing a large, multicolor flow cytometry panel, FluoroFinder has developed a range of resources to help. Our Antibody Search function lets you find flow cytometry-validated antibodies for your target antigens, while our Spectra Viewer allows you to compare the properties of over 1,000 fluorophores in a single resource. For panel design, use our Panel Builder to view the fluorophore and antibody offerings of >60 suppliers, including an extensive selection of viability dyes for controlling your flow cytometry experiment.
Sign up for our eNewsletter to receive further updates about flow cytometry and other fluorescence-based techniques.