Understanding the advantages and disadvantages of different immunodetection strategies is important to achieve accurate results.
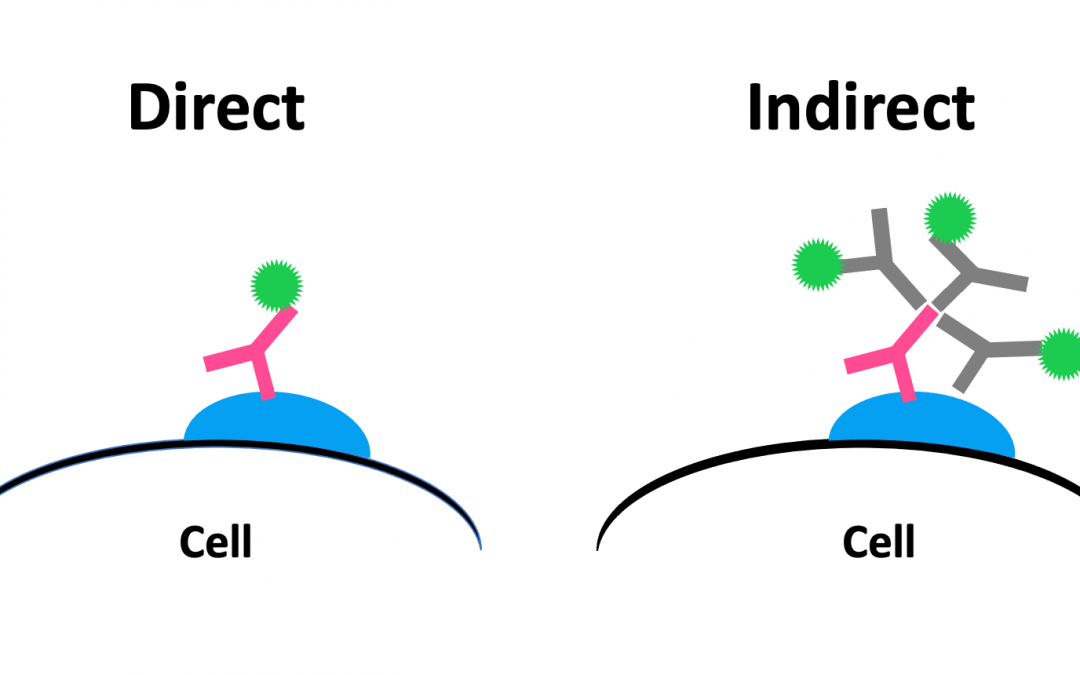
Microscopy-based techniques such as immunocytochemistry (ICC) and immunohistochemistry (IHC) often use fluorophore or enzyme-labeled antibodies for detecting targets of interest. The labeled antibodies can be specific to the analyte of interest (direct detection) or raised against the species and immunoglobulin class/sub-class of the primary antibody (indirect detection).
Direct Detection: Advantages and Disadvantages
During direct detection, labeled primary antibodies are used to visualize the target of interest. A major advantage of this approach is that it shortens the experimental workflow by eliminating the need for a secondary antibody incubation step and any associated washes. Direct detection also allows for multiplexing of antibodies sharing the same host species and minimizes the risk of non-specific signal due to secondary antibody cross-reactivity.
However, signals obtained by direct detection often appear weaker in comparison to those obtained via indirect methods because there is no means of signal amplification. “Notably, the signal may be limited by the affinity of the primary antibody, if monoclonal, as monoclonals target only one epitope,” notes Miranda Lewis, Ph.D., Marketing Manager at Jackson ImmunoResearch. In addition, direct detection is often more expensive since labeled primary antibodies typically have a higher price tag than their unlabeled counterparts. The availability of labeled primary antibodies may also limit flexibility for experimental design, although antibody labeling is now relatively straightforward to perform in-house.
“Our ReadiLink™ Antibody Conjugation Kits let you quickly conjugate microscale volumes of antibody (50-100 µg) to our ReadiLink™ Dyes,” says Sharon Sanderson, Ph.D., Global Product Manager at Bio-Rad Laboratories. “You simply add your antibody to the vial of dye included in the kit and incubate for an hour at room temperature, before quenching any unbound dye with the provided quenching reagent. Your labeled antibody is then ready to use.” For even faster antibody labeling, Bio-Rad offers LYNX Rapid Plus Conjugation Kits® that have an incubation time of just 15 minutes.
Indirect Detection: Advantages and Disadvantages
Indirect detection uses unlabeled primary antibodies to recognize and bind the target of interest in addition to labeled secondary antibodies to produce a measurable signal. “This provides signal amplification in two ways,” reports Lewis. “Firstly, the secondary antibody, if polyclonal, can recognize multiple sites on the primary antibody. Additionally, the secondary antibody can be conjugated to multiple reporter molecules without compromising target protein recognition. Signal amplification is especially useful when detecting low abundance proteins.” Indirect detection also provides access to a wider range of labels, allowing researchers to design experiments with greater flexibility.
While the indirect method offers superior signal, researchers have expressed concern about the need to perform an additional incubation step. To address this, Jackson ImmunoResearch has developed FabuLight™ secondary antibodies that are available with a choice of different fluorophores or biotin. “FabuLight antibodies are Fab fragment secondary antibodies specific to the Fc region of IgG or IgM primary antibodies,” explains Lewis. “They can be used to label primary antibodies prior to incubation with the sample, thereby removing the need to perform two separate antibody incubation steps.”
Using secondary antibodies also increases the risk of cross-reactivity with species other than the target. While this can be problematic when multiplexing, this risk can be mitigated by setting up the multiplexed experiment correctly and choosing secondary antibodies with minimal cross-reactivity with other species in the assay. “Because immunoglobulins from different species often share similar structure and homology, secondary antibodies raised against the immunoglobulins of one species may also recognize those of another species,” clarifies Lewis. “Using cross-adsorbed secondary antibodies is necessary to circumvent this issue, although researchers should keep in mind that antibodies which are cross-adsorbed against a species which is closely related to that of their primary antibody may result in diminished signal.”
| Direct Detection | Indirect Detection | |
| Advantages |
|
|
| Disadvantages |
|
|
Alternative Approaches
Researchers have developed alternative methods for detecting targets of interest. For example, combining a biotinylated primary antibody with a labeled streptavidin reagent is another option when it comes to experimental design. “Labeled streptavidin reagents offer excellent signal amplification,” adds Lewis. “By incubating samples with an unlabeled primary antibody, followed by a biotinylated secondary antibody, and then adding a labeled streptavidin reagent, researchers may be able to increase their chances of detecting scarce targets.”
Another common tactic is to use F(ab’)2 fragments instead of whole antibodies for applications such as IHC where background signal can result from Fc receptor binding. “Fc receptors are found on the surface of cells such as monocytes, macrophages, natural killer (NK) cells, and B cells and can bind antibody reagents via the Fc region,” reports Sanderson. “Because F(ab’)2 fragments lack an Fc region, they do not undergo this type of unwanted interaction.” In addition, the F(ab’)2 fragments’ smaller size can enhance tissue penetration.
Supporting Your Research
Whichever detection method you select for your research, FluoroFinder has resources for you. Our Antibody Search function allows you to find and discover antibodies from all suppliers for your targets of interest, and our Spectra Viewer allows you to visualize over 1,000 fluorophores in the context of your microscope configuration and filter through conjugated antibodies by application, citation count, and even price.
Sign up for our eNewsletter to receive further updates on microscopy and many other research techniques.