Reducing autofluorescence is critical in fluorescence-based research
Techniques such as fluorescence microscopy, flow cytometry, and western blotting often rely on the use of fluorophore-labeled antibodies. The main reason for this is that fluorescence-based detection allows for multiplexing, which conserves limited sample material and researchers’ time. However, to achieve reliable results from these types of experiments, it is critical to use protocols designed to minimize autofluorescence. Autofluorescence can not only be mistakenly identified as the target of interest, but also obscure the detection of low abundance analytes. Here, SouthernBiotech and Vector Laboratories suggest strategies to minimize autofluorescence to improve the accuracy of experimental results.
What is Autofluorescence?
Autofluorescence is the fluorescent signal emitted by biological samples originating from sources other than the fluorophore-labeled antibodies used for target detection. “Autofluorescence is often endogenous to the sample itself,” reports Leslie Bibb, Technical Services Manager at SouthernBiotech. “For example, collagen, riboflavin, and NADH all autofluoresce, as do elastin, the lipofuscins, and the heme groups present in red blood cells. In addition, autofluorescence can be a result of sample processing. Aldehyde fixatives such as formalin and glutaraldehyde are well known for generating autofluorescence, as are many of the nitrocellulose membranes used for western blotting.”
How to Determine Whether Autofluorescence Will Present Challenges
The easiest way to determine whether autofluorescence will present technical issues is to run an unlabeled control. This involves treating your sample per usual, but omitting labeled antibody reagents. Upon detection, the measured fluorescence can be attributed to the sample or other assay components. This serves as a starting point to address the problem.
How to Reduce Autofluorescence
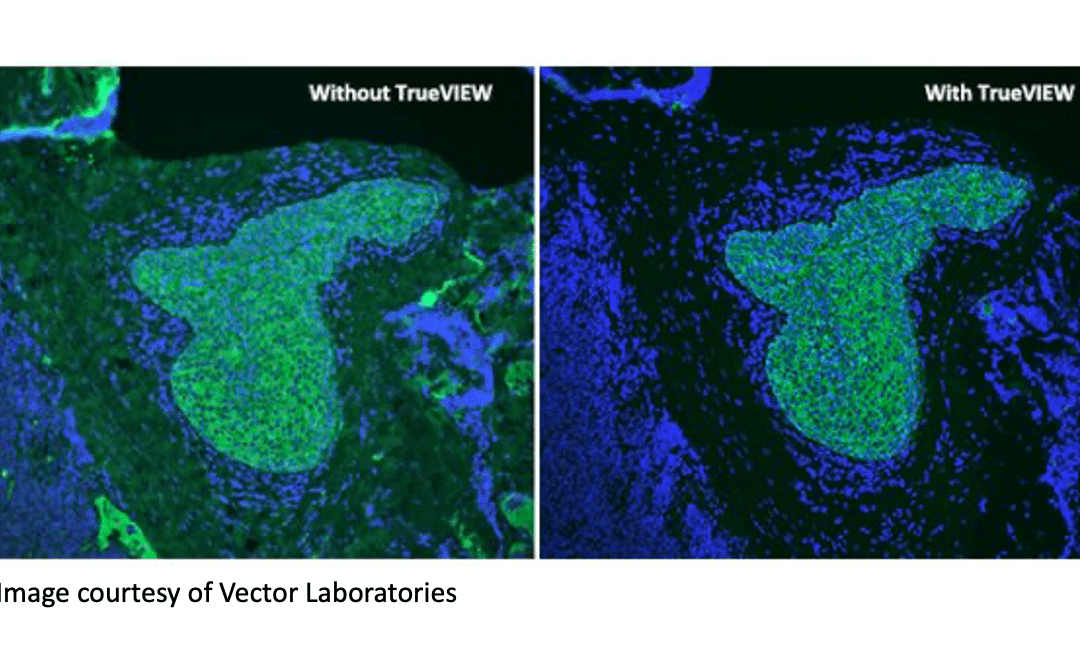
There are multiple strategies to reduce autofluorescence. Erika Leonard, Director of R&D at Vector Laboratories, explains that because autofluorescence often occurs in the blue to green spectrum (350 – 550 nm), a common tactic is to select fluorophores that emit in the red to far-red region (620 – 750nm). “If researchers are experiencing problems linked to autofluorescence, we often suggest they try switching from, say, a DyLight™ 488 conjugate to a DyLight™ 649 conjugate,” she says. “We also offer our Vector® TrueVIEW® Autofluorescence Quenching Kit as a unique means of diminishing unwanted autofluorescence from non-lipofuscin sources. This works by binding and effectively quenching the autofluorescent elements in various sample types, including problematic tissues such as kidney, spleen and pancreas.”
In addition to these approaches, there are several other methods to minimize autofluorescence:
-
Change the Fixation Method
Aldehyde fixatives produce autofluorescence by forming Schiff bases through reacting with amine groups. Although using paraformaldehyde instead of glutaraldehyde for fixing samples or decreasing the concentration of paraformaldehyde and exposure duration can reduce autofluorescence, these methods are often not sufficient. “An alternative solution is to try replacing the aldehyde-based fixative with an organic solvent such as ice-cold ethanol or methanol,” suggests Bibb. “If this is not possible, treating samples with sodium borohydride diluted in a physiological buffer such as PBS or TBS is a common method for reducing aldehyde-based autofluorescence.”
-
Remove Red Blood Cells
Red blood cells transport oxygen by capturing it from the lungs via iron-containing heme groups in hemoglobin. However, the polyphyrin ring structure of heme groups is a major source of autofluorescence, so it is important to remove red blood cells from samples when possible. “If you are working with whole blood, red blood cells can easily be removed by lysis,” says Leonard. “Performing adequate wash steps is essential, however, to ensure that all of the lysed contents are eliminated.”
Bibb adds that, for tissue samples, perfusing with PBS prior to fixation will remove red blood cells. However, this is not feasible when working with post-mortem samples, which are more commonly used. “In this situation, researchers may wish to introduce an autofluorescence-reducing treatment into their experimental workflow,” she says. “Methods based on the use of ultraviolet (UV) light, ammonia, copper sulfate, Sudan Black B, sodium borohydride, or Trypan Blue are all widely cited in the literature.”
-
Eliminate Dead Cells and Debris
Dead cells are generally more autofluorescent than live cells and release significant amounts of autofluorescent debris. Thus, eliminating dead cells from samples is especially important in flow cytometry. Increased non-specific antibody binding to dead cells often leads to false positives and overlooking low abundance populations. Dead cells can be removed from suspension samples by low speed centrifugation or with the use of a Ficoll gradient. In addition, researchers often include a viability dye in staining panels to gate out dead cells before identifying other cellular populations.
-
Reduce the Concentration of FBS in the Staining Buffer
Staining buffers often include a low concentration of protein, typically fetal bovine serum (FBS), to block non-specific antibody binding. However, FBS absorbs in the violet to blue spectrum, increasing autofluorescence. To address this, researchers can use a different protein source, such as bovine serum albumin (BSA), or reduce the concentration of FBS without compromising its blocking efficacy. “For live-cell imaging, a medium that is free of both FBS and phenol red – another source of autofluorescence – may be preferred, but you should always perform appropriate testing with your cells to ensure that there are no unwanted phenotypic changes,” cautions Bibb.
-
Carefully Select Fluorophores
Fluorophore selection is key to minimizing autofluorescence. In addition to selecting dyes spectrally distinct from the observed autofluorescence, researchers may want to consider fluorophores with narrow excitation and emission spectra that are easily distinguishable from the background. “Selecting brighter fluorophores such as phycoerythrin (PE) or allophycocyanin (APC) can reduce the impact of autofluorescence on results interpretation,” comments Leonard. “It is also recommended that researchers titrate fluorophore-based reagents to maximize the signal-to-background ratio.”
Supporting Your Research
Whichever fluorescence-based technique you are performing, FluoroFinder is here to help you with the reagent selection process. You can visualize the excitation and emission spectra of fluorophores compatible with your experiment on our Spectra Viewer to help select fluorophores distinguishable from your background, or browse the spectral properties of dyes in our Dye Database comprising of over 1,100+ fluorophores.
In addition, our partners have developed various autofluorescence resources. View a recent Vector Laboratories’ webinar on overcoming background interference by clicking the ‘webinars’ tab on this page, and take a look at SouthernBiotech’s latest autofluorescence blog.
Sign-up for our eNewsletter to receive regular updates about a broad range of fluorescence-based research techniques and discover the latest fluorophore offerings from our partners.