Understanding the biological density of proteins or antigens expressed by each individual cell is an imperative component of all cell-based analytical methods. The comparative change in protein expression, also called antigen density, is indicative of the essential molecular underpinnings of disease, therapeutic efficacy, activation, and senescence. In flow cytometry, to build the most accurate, internally consistent panel, you must begin with an understanding of not only the expression of a single antigen but the combinations of all antigens expressed or absent on a single cell. For the majority of applications, this translates to the wider heterogeneity of different cells in a tissue or patient sample and the relationship of their expression level and function to each other.
Determining Antigen Density
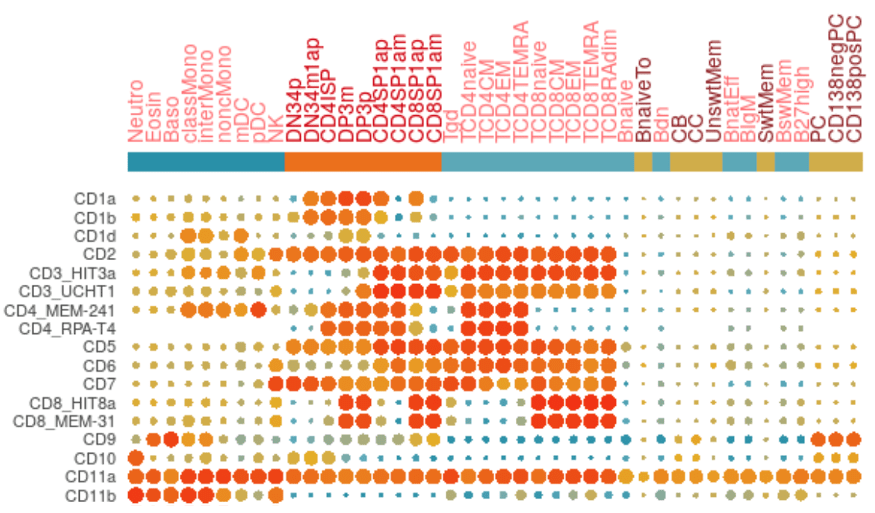
With methods and cell analysis platforms that expand the number of parameters and cellular phenotypes able to be derived from a single heterogeneous sample, antigen density quantitation on specific cell subsets is well explored in the literature. The efforts of the Human Cell Differentiation Molecules (HCDM) consortium are at the forefront of standardizing antigen expression on healthy humans and making it quantitative with internal standards to enable future comparison with data derived from other groups and different biological contexts. There are many journal articles resulting from these efforts over the years including “Standardization of Workflow and Flow Cytometry Panels for Quantitative Expression Profiling of Surface Antigens on Blood Leukocyte Subsets: An HCDM CDMaps Initiative” (Kuzilkova D, et al (2022). Frontiers in Immunology 13: 827898). Another important effort employs mass cytometry (CyTOF) in a similar effort in 2019 by Adeeb Rahman and El-Ad David Amir analyzing 350 antibodies in this journal article: “Development of a Comprehensive Antibody Staining Database Using a Standardized Analytics Pipeline” (Frontiers in Immunology 10:1315). However, antigen expression is unique to cell type, disease state, and model organism, so we suggest conducting an extensive literature search prior to designing any experiment.
If there is not a comprehensive overview of antigen density for the cell subset, tissue type, disease state, or model organism that interests you, there are ways for each researcher to contribute to the collective efforts of antigen density standardization. Here are the important components to make data relevant to this effort:
- To employ a standardization control – Standardization controls like Phycoerythrin QuantibriteTM beads from BD Biosciences help relate the resulting MFI values to a transferable metric like the number of PE molecules.
- To employ the same phenotypic markers and ontologic gating strategy in order to compare expression levels on the same derived subpopulations – Biolegend also sells a kit that can enable this sort of application on a larger scale in mouse and human called LegendScreenTM where 252 mouse and 371 human antibodies respectively are conjugated to PE and inserted into a flow cytometry panel with additional markers that outline cell subsets. This nod to the implementation of standardizing elements in all flow cytometry experiments has not only the potential to dramatically improve the depth of our understanding of the content, but also dramatic implications to improve experimental reproducibility in biomedical research.
Antigen Density in Flow Cytometry Panel Design
Antigen density correlates directly to the resultant fluorescent signal intensity and needs to be a primary consideration when selecting fluorophores to be paired with antigens within a flow cytometry experiment. In general, bright fluorophores should be paired with antigens with low expression and dim fluorophores with highly-expressed antigens. This ensures that the MFI signal has the best chance to remain on-scale and within the linear detection range of the photomultiplier tube (PMT) or avalanche photodiode (APD) detector.
FluoroFinder’s Panel Builder streamlines the panel design workflow by enabling researchers to filter fluorophores to match their antigens’ expected expression levels. Researchers can manually set relative antigen densities for each of their markers to limit suggested fluorophores to only those compatible with their marker’s antigen expression within each channel. In addition, our new premium automated panel design tool, IntelliPanel will automatically suggest fluorophores for a marker combination based on conditional inputs like antigen density.
Antigen Density Resources
Many antibody suppliers have published antigen expression levels of various markers for common cell types. These resources are often useful starting points for researchers studying markers known to identify a specific cell type or those unfamiliar with the antigen densities of their chosen markers.
Here are some of our favorite resources for determining relative antigen expression levels:
- CDMaps: Dynamic Profiling
- Cell Markers – BioLegend
- Essential Markers – BioLegend
- Expression of Common Surface Markers on Blood Cells – BioLegend
- Human Cell Marker Selection Tool – Bio-Rad
- MACS Handbook – Miltenyi Biotec
- Human CD & Other Cellular Antigens – ThermoFisher Scientific
- Human CD Antigen Chart – Abcam
- Human CD Antigen Guide – Abcam