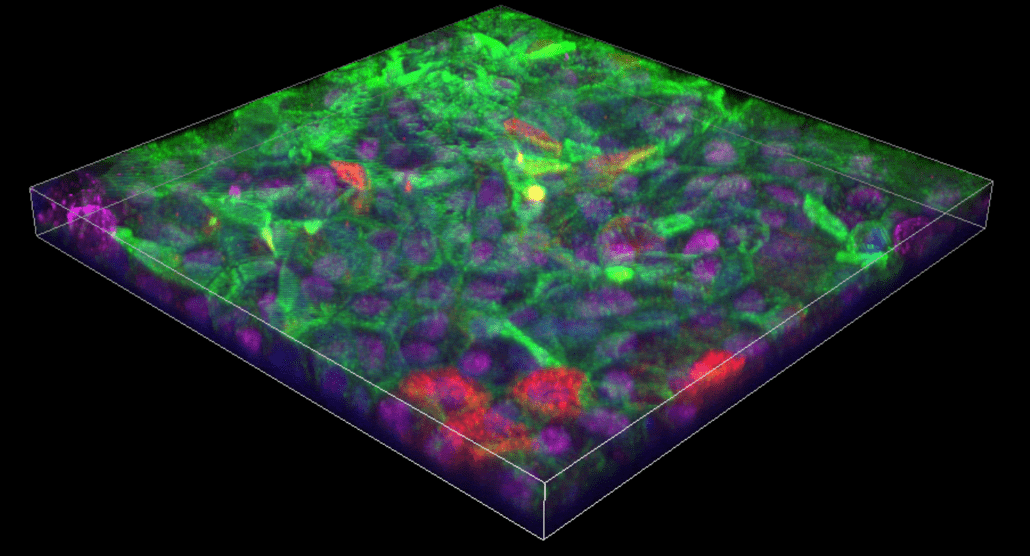
Advanced microscopy techniques enable deeper imaging
Advanced microscopy platforms are becoming more widespread for the depth of information they provide. Among these newer modalities, confocal microscopy has risen in popularity for imaging thick fluorescently-labeled specimens. Super-resolution microscopy (SRM), another recently developed technique, supports fine mapping of structures such as microtubule protofilaments and subcellular organelles. In this article, we explain the basics of confocal microscopy and SRM and overview their advantages for scientific research.
What is Confocal Microscopy?
Conventional widefield fluorescence microscopes illuminate an entire sample with light of a specific wavelength, exciting the fluorophores within it. This means that any fluorescence emitted away from the region of interest can interfere with the resolution, making imaging of thicker samples (> ~2 µm) particularly challenging. Confocal microscopy, first patented by Marvin Minsky in 1957, eliminates unwanted secondary fluorescence by combining point illumination of the focal plane of interest with detection via a pinhole in an optically conjugate plane in front of the detector. This ensures that only light from the region of interest is detected and improves resolution.
Advanced Confocal Microscopy Techniques
Several adaptations of the confocal microscope have been developed, of which the laser scanning confocal microscope (LSCM) is the most widely used. A typical LSCM uses high-speed oscillating mirrors to rapidly pass a laser beam across the sample in a raster pattern. One mirror moves the beam along the x-axis, while the other moves it along the y-axis within the same focal plane, generating an image of the sample from the fluorescence detected at each point. By scanning multiple focal planes, it is possible to produce a 3D image.
The spinning disk confocal microscope features hundreds of pinholes on a rotating disk to increase image acquisition speeds and reduce photobleaching. A variation of this technique, the dual spinning disk microscope, employs similar principles but incorporates a second disk to increase the amount of light directed into each pinhole and achieve higher sensitivity. In addition, various programmable array microscopes (PAMs) have been developed, which feature complex optics and detectors to provide faster and/or higher resolution imaging.
What is Super-Resolution Microscopy?
The term super-resolution microscopy encompasses a growing number of microscopy techniques developed to overcome the optical resolution limit. This is described by Abbe’s law, which states that resolution is equal to λ/(2NA), where λ is the wavelength of light used to illuminate the sample and NA is the numerical aperture of the microscope’s objective. In theory, the lower the wavelength and the larger the numerical aperture, the more detailed the image. However, in practice, the lateral resolution of conventional optical microscopes is limited to around 200 nm (and the axial resolution to around 500 nm), primarily due to light being diffracted as it passes through the objective
Overcoming Optical Resolution Limits with SRM
Super-resolution microscopes overcome the optical resolution limit in one of two ways. The first involves reducing the size of the point spread function (PSF), or the point of light and any surrounding blurring (due to diffraction) emitted by a fluorophore. Reversible Saturable Optical Linear Fluorescence Transitions (RESOLFT), Stimulated Emission Depletion Microscopy (STED), Ground-State Depletion Microscopy (GSD), and Saturated Structured Illumination Microscopy (SSIM) all use this approach. To learn more about these techniques, see our Fluorophores for Super-Resolution Microscopy blog.
The second approach involves sequentially visualizing small quantities of fluorophores, resulting in the emission of light at distinct time intervals. Photo-Activated Localization Microscopy (PALM), Stochastic Optical Reconstruction Microscopy (STORM), and direct STORM (dSTORM) all function this way. Like the SRM techniques mentioned above, these methods often rely on the use of photoswitchable fluorophores. With SRM, researchers can attain resolution of as little as 10 nm, which will likely decrease as the technology evolves.
Which Microscopy Technique Should I Choose?
Both confocal microscopy and SRM each have advantages and disadvantages. Advantages of LSCM, the most common form of confocal microscopy, include its accessibility, ease of use, and capacity for live-cell imaging. However, LSCM is limited by its optical resolution limit and relatively slow scanning speed, which increases a sample’s susceptibility to photobleaching. . Although SRM can generate images with superior resolution, the cost and difficulty of implementing this technique is currently unsuitable for imaging live cells. Deciding which microscopy technique largely boils down to the aim of your research and your access to the technology and expertise. Manufacturers can provide practical guidance for platform selection.
Supporting Microscopy-Based Research
Whichever microscopy technique you’re using, FluoroFinder has a variety of resources to help. Our Spectra Viewer helps you compare over fluorophores validated for microscopy from all major suppliers on a single intuitive platform, and streamlines the antibody selection process.
Sign up for our eNewsletter to receive updates on fluorophores for confocal microscopy, SRM, and other fluorescence-based microscopy techniques.