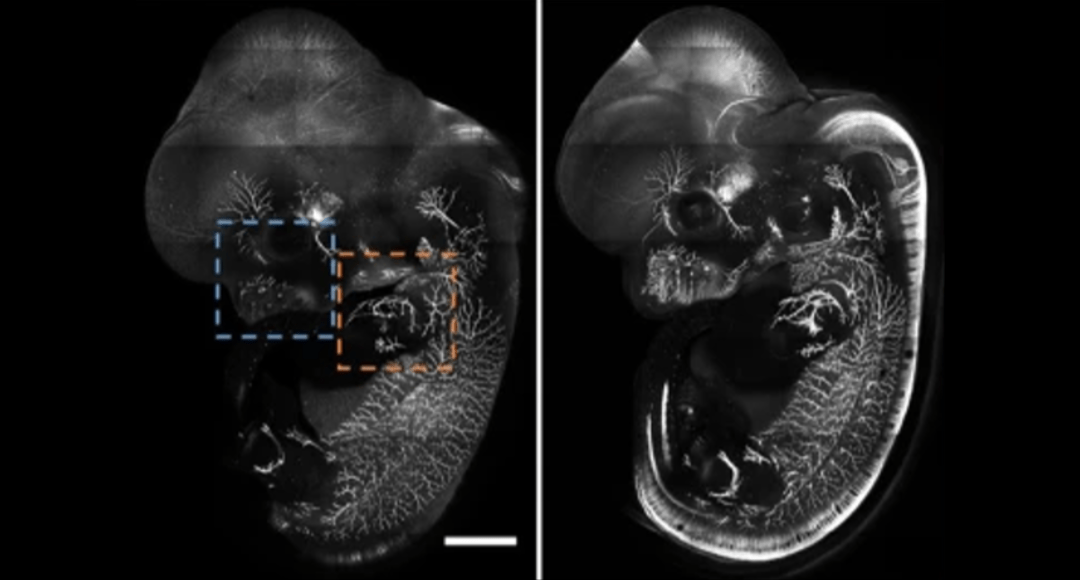
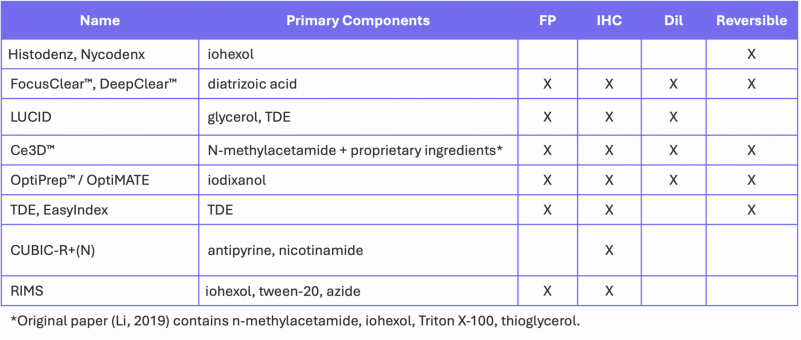
Cover Image: E12.5 whole mouse embryo was immunostained with neurofilament antibody and cleared with RTF. Scale bar, 1000 μm. Used without modification under Creative Commons Attribution 4.0 International License from (Yu T. Z., 2018) Figure 4a.
As explained in last month’s article, tissue clearing techniques enable fluorescent imaging deep within thick samples using methods such as light-sheet microscopy, confocal and multiphoton. It is achieved by minimizing refractive index (n) differences within the sample which cause cloudiness and photon scattering. Solvent-based dehydration methods largely replace water (n = 1.33) with higher n organic molecules, while the hydrophilic methods discussed here increase the n of the aqueous phase and/or reduce the contribution of other biomolecules (n = 1.4-1.65) to achieve clearing and increase transparency.
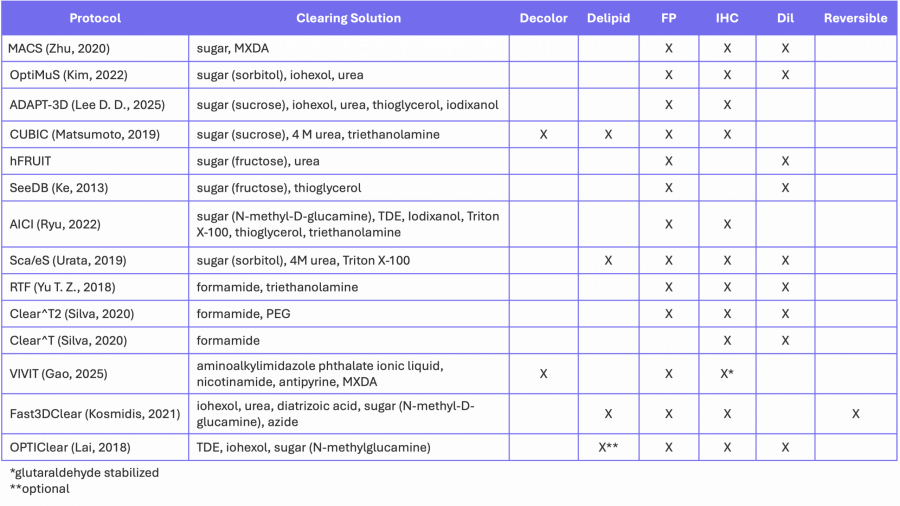
In many ways, hydrophilic protocols are simpler than solvent-based dehydration methods because they avoid serial dehydration steps, but they tend to be quite slow because many index-matching solutions have high viscosity, reducing the diffusion rate into tissue. Table 1 summarizes a number of commonly used aqueous clearing protocols, the major reagents used for index-matching and the types of fluorescent signals that can be detected using each method.
Hydrophilic (Aqueous) Methods
Generic protocol:
- Fixation
- Optional decalcification
- Optional hydrogel embedding (described in the next section)
- Optional decolorization and detergent-based delipidation
- Optional Labeling and IHC
- Index-matching and imaging
Table 1. Aqueous-based clearing methods.
Similar to the solvent-based dehydration methods described previously, fixation typically proceeds via PFA diffusion or perfusion (in whole animals). Optional decalcification is performed using EDTA solution.
Decolorization and Delipidation
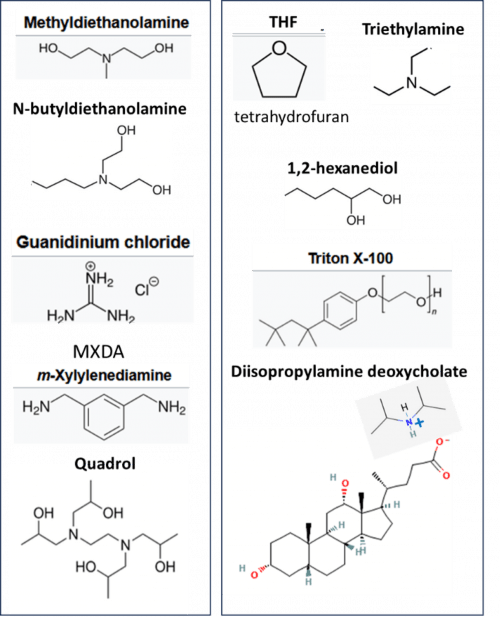
Decolorization and delipidation are often performed together in a single step using reagents shown in Figure 1 as detailed below.
Amino alcohols (Figure 1, left) such as N-methydiethanolamine (ADAPT-3D), N-butylethanolamine (CUBIC-L, ADAPT-3D), and Quadrol (CUBIC), help to bind heme, the major chromophore in tissue (especially liver, kidney and muscle). Low concentrations of urea (CUBIC), guanidium chloride (Sca/eS) and MXDA (MACS) help to denature and solubilize membrane proteins.
High concentrations of the detergents Triton X-100 (CUBIC, Sca/eS) or PEG-4-octylphenyl ether (CUBIC-L) are used for delipidation (Figure 1, right). PEG (the hydrophilic portion of Triton X-100) is known to stabilize proteins. (Arakawa, 2017) Other methods utilize the water-miscible solvent THF with amphiphilic 1,2-hexanediol (ADAPT-3D) or the surfactant triethylamine (Fast3DClear) to dissolve lipids. The newly described VIVIT method (Gao, 2025) uses the surfactant diisopropylamine deoxycholate to delipidate the sample with smaller micelles than Triton X-100; increasing the diffusion rate.
Several protocols skip the delipidation and decolorization step, including BioLegend’s Ce3DTM, Photon Tech Innovations’ LUCID, the ClearT methods, SeeDB, AICI, OptiMuS and MACS. The fixed and labeled samples are immersed directly into index-matching solution or in a series of increasing concentrations of index-matched solutions (RTF, ClearT methods). Many of these methods preserve cell membranes so neuronal-tracing dyes, like DiI, can be used (Table 1).
Figure 1. Reagents used for decolorization (left) and delipidation (right) in the aqueous clearing protocols.
Labeling and IHC
Labeling and IHC proceed as usual- diffusion can be quite slow in large samples, so permeabilization steps are often required. BioLegend’s Ce3DTM tissue clearing kit includes both a Permeabilization/ Blocking Buffer and Ce3D Ab Diluent Buffer, specifically for IHC staining. A method called PRESTO (Lee E. , 2016) uses centrifugal pressure or convective flow to increase diffusion rates of antibodies into thick tissues to speed up IHC labelling of thick tissues.
Index-Matching
Index-matching is performed with a number of different high n reagents (Figure 2) in aqueous buffer. The majority of methods employ concentrated sugar solutions (MACS, OptiMuS, ADAPT-3D, CUBIC, hFRUIT, SeeDB, AICI, Sca/leS, Fast3DClear and OPTIClear). Alcohols, polyols (including sugars) and PEG have very high aqueous solubility and have been shown to stabilize proteins (Arakawa, 2017).
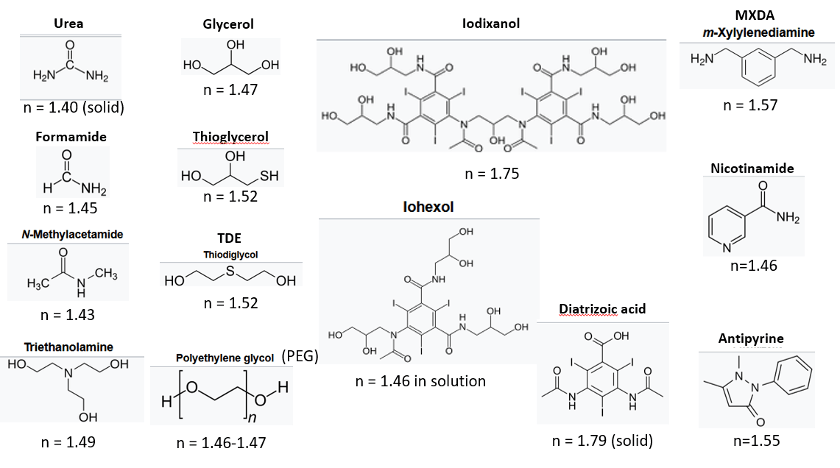
Figure 2. Chemicals used in aqueous index-matching solutions.
Large heteroatoms (S, I) increase the polarizability and n of the reagents. Compounds like 2,2’-thiodiethanol (TDE – Figure 2) and thioglycerol preserve all sample components, including hemoglobin, other pigments and fluorescent proteins, as well as lipids, but at concentrations >70%, fluorescent dyes can become unstable. The iodated reagents, iohexol, iodixanol and diatrizoic acid are FDA-approved X-ray contrast agents with very high n, high water solubility and low toxicity. They are used alone or to reduce viscosity (Treweek, 2015), especially in commercially available index-matching solutions (Table 2). They have also been used in live-tissue clearing. (Boothe, 2017)
At low concentrations, formamide, urea and, to a lesser extent, N-methylacetamide, help improve permeability of tissues, while at high concentrations they denature proteins, depolymerize f-actin and cause hydrogen-bond disruptions in C-G rich regions of DNA. (Arakawa, 2017) The “hyperhydration” protocols (Sca/eS, ClearT, hFRUIT, CUBIC) exploit the hydrogen-bond forming ability of amides, amines and alcohols to disrupt the superstructure and reduce n of collagen fibers (Yu T. Z., 2021) leading to tissue swelling.
The VIVIT (Gao, 2025) protocol requires pretreatment of the sample with sodium octyl sulfate to ionize biomolecules before incubation with the ionic liquid-based index-matching solution. An advantage of this technique is its lack of shrinking or swelling and its ability to be frozen in a non-crystalline glassy state for long-term tissue storage.
Table 2. Commercially available index-matching agents.
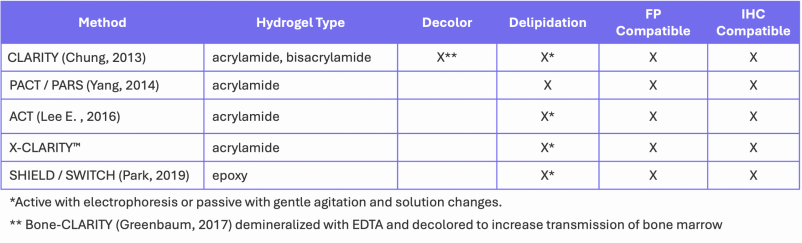
Hydrogel Embedding
The methods above provide good clearing, but the removal of so many structural elements makes the samples fragile. Combination methods have been described where tissue was cleared with CUBIC (Richardson A. F.-A., 2022) or the solvent-based DISCO method (Nudell, 2022) before hydrogel embedding for mechanical stabilization. Other techniques (Table 3) anchor biomolecules to hydrogel matrices before washing away the lipids. The two common hydrogels are acrylamides (CLARITY, PACT, PARS, ACT, X-CLARITYTM) and epoxies (SHIELD), both of which react with nucleophilic moieties including thiol, amine and alcohol groups to covalently link biomolecules to the hydrogel matrix, forming a tissue/hydrogel hybrid.
Some methods (SHIELD) utilize a SWITCH protocol which involves diffusing monomers into the tissue before initiating the polymerization step by pH, temperature, initiator diffusion, or a combination. (Murray, 2015) This speeds up polymerization and leads to a more consistent matrix. SWITCH can also be used for the initial fixation step and for multiple rounds of Ab labeling.
Table 3. Hydrogel embedding methods.
Hydrogel-based methods do not have an explicit decolorization step, but SDS, or other detergents (LifeCanvas’ delipidation buffer), is used for delipidation, denaturing proteins and releasing chromophores. Delipidation/decolorization can either proceed actively by electrophoresis through the hydrogel using a device like Life Canvas Tech’s SmartBatch+, SmartClear II Pro or Logos Biosystems’ X-CLARITYTM, or passively via diffusion (PACT) and perfusion (PARS). Traditional neuronal lipid markers, such as DiI, cannot be used with hydrogel methods, but linkable analogs are available. (Jensen, 2016) Commercially available index-matching solutions (Table 2) are often used for imaging of tissue/hydrogel hybrids.
Performance
Researchers need to weigh several factors when choosing the clearing protocol, including processing time, ease of preparation, preservation of size and preservation of signal.
A major difference between the protocols described above is the processing time. Many of the solution methods in Tables 1 and 2 are quite slow due to high viscosity of concentrated sugar or urea solutions, but these reagents are cheap, widely available and easy to prepare. Diffusion into thick tissues can take several days to months, so researchers optimize processing times with thinner sections (~ 1 mm) before working up to whole mounts. In general, the perfusion-based methods (Yang, 2014) and those using less-viscous solutions (iodated reagents) clear faster – especially for whole organs or animals. While active electrophoretic delipidation only takes a few hours to clear fixed tissue before (FxClear (Choi, 2019), Binaree tissue clearing kit) or after hydrogel embedding, it requires extra equipment.
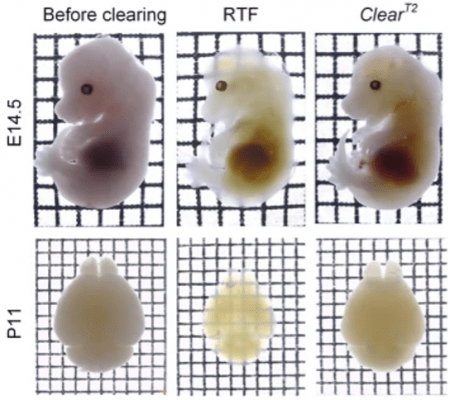
Figure 3. Whole mouse embryos (E14.5) and neonatal (postnatal day 11, P11) whole-brain samples cleared with RTF and ClearT2 overnight (transmission images). Grid size, 1.45 mm × 1.45 mm. Used without modification under Creative Commons Attribution 4.0 International License from (Yu T. Z., 2018) Figure 1b.
Like the dehydration methods described last month, some methods cause sample shrinkage (MACS, RTF), while others (OptiMuS, SeeDB (Figure 4),ClearT2 (Figure 3)) maintain sample size. Hyperhydration methods (CUBIC, Sca/eS) lead to swelling and mechanical destabilization (Figures 3,4). In contrast, the size of hydrogel-cleared samples (CLARITY, SHIELD) can be reversibly controlled by the osmolarity of the index-matching solution. This property is exploited in expansion microscopy applications (e-PACT) and MAP techniques (Lee M. , 2021)
The aqueous methods generally do a better job of preserving fluorescent proteins compared to the dehydration methods, although high TDE concentrations have been shown to degrade fluorescent protein signal over time. IHC works well in these methods, presuming antigens are not washed away during detergent-mediated delipidation steps. Epitope masking can also occur in hydrogel-based methods during the conjugation process, so control experiments are required to ensure proteins of interest are still present and accessible. Diffusion of IHC reagents can be very slow, but techniques like permeabilization, transient expansion, or electrophoretic methods can speed the labeling process. Many methods are also compatible with DiI membrane labeling (Tables 1-2).
For some researchers, long-term sample preservation is a concern, especially for human tissue-bank samples. Many commercially available clearing agents (Table 2) are reversible so samples can be returned to their storage medium after imaging. Other options include hydrogel-tissue hybrids that provide long-term structural stability, and the VIVIT protocol which enables crystal-free sample freezing. (Gao, 2025)
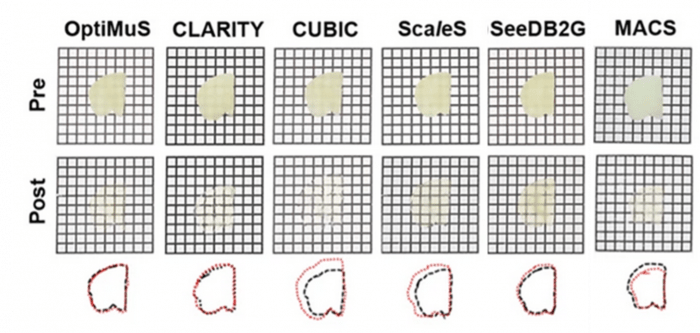
Figure 4. Bright-field images of pre- and post-cleared 1 mm thick rat brain samples using various methods. Overlapped images of outlined pre- and post-cleared brain tissues (black: pre-, red: post-cleared). Grid size = 1.5 × 1.5 mm. Excerpt used without modification under Creative Commons Attribution 4.0 International License from (Kim, 2022) Figure 1b.
While there is no “perfect” clearing method suitable for all applications, new methods are constantly being developed to find the perfect mix of speed, ease of use, cost, signal preservation and sample longevity. Until then, researchers will have to weigh these factors when choosing a protocol. For additional reading on this active research topic see (Richardson D. G., 2021) (Yu T. Z., 2021), (Choi, 2019) (Richardson D. L., 2016)
Supporting Your Research
As tissue clearing and advanced 3D fluorescence imaging methods continue to expand research possibilities, having the right experimental tools is essential. FluoroFinder supports your work with an integrated Microscopy Spectra Viewer for comparing fluorophores under solvent- and instrument-specific conditions. With access to the most comprehensive fluorophore database and antibody search tools, FluoroFinder helps streamline experiment planning so you can focus on generating clear, reproducible results from your cleared tissue imaging studies.
References:
- Arakawa, T. (2017) doi:10.1007/s12551-017-0339-6
- Boothe, T. H. (2017) doi:10.7554/eLife.27240
- Choi, Y. L. (2019) doi:10.5607/en.2019.28.3.436
- Chung, K. (2013) doi:10.1038/nature12107
- Gao, Y. X. (2025) doi:10.1016/j.cell.2025.07.023
- Greenbaum, A. C. (2017) doi:10.1126/scitranslmed.aah6518
- Jensen, K. B. (2016) doi:10.1038/srep32674
- Ke, M.-T. F. (2013) doi:10.1038/nn.3447
- Kim, K. N.-S. (2022) Communications Biology, 5, 431
- Kosmidis, S. N. (2021) doi:10.1016/j.crmeth.2021.100090
- Lai, H. L. (2018) doi:10.1038/s41467-018-03359-w
- Lee, D. D. (2025) doi:10.21203/rs.3.rs-6109657/v1
- Lee, E. (2016) doi:/10.1038/srep18631
- Lee, M. (2021) doi:10.1038/s41598-021-02632-1
- Li, W. G. (2019) doi:10.1038/s41596-019-0156-4
- Matsumoto, K. M. (2019) doi:10.1038/s41596-019-0240-9
- Murray, E. C.-Y.-G.-Y. (2015) doi:10.1016/j.cell.2015.11.025
- Nudell, V. W. (2022) doi:10.1038/s41592-022-01427-0
- Park, Y.-G. S.-C. (2019) doi:10.1038/nbt.4281
- Richardson, A. (2022) doi:https://doi.org/10.3390/gels8010032
- Richardson, D. (2021) doi:10.1038/s43586-021-00080-9
- Richardson, D. (2016) doi:https://doi.org/10.1016/j.cell.2015.06.067
- Ryu, Y. K.-J.-J. (2022) doi:10.3390/ijms23126826
- Silva, D. C.-D. (2020) doi:/10.3390/ijms22010266
- Treweek, J. C. (2015) doi:10.1038/nprot.2015.122
- Urata, S. I.-Y. (2019) doi:10.21769/BioProtoc.3342
- Yang, B. T.-K. (2014) doi:10.1016/j.cell.2014.07.017
- Yu, T. Z. (2018) doi:10.1038/s41598-018-20306-3
- Yu, T. Z. (2021) doi:10.1016/j.isci.2021.102178
- Zhu, J. Y. (2020) doi:10.1002/advs.201903185