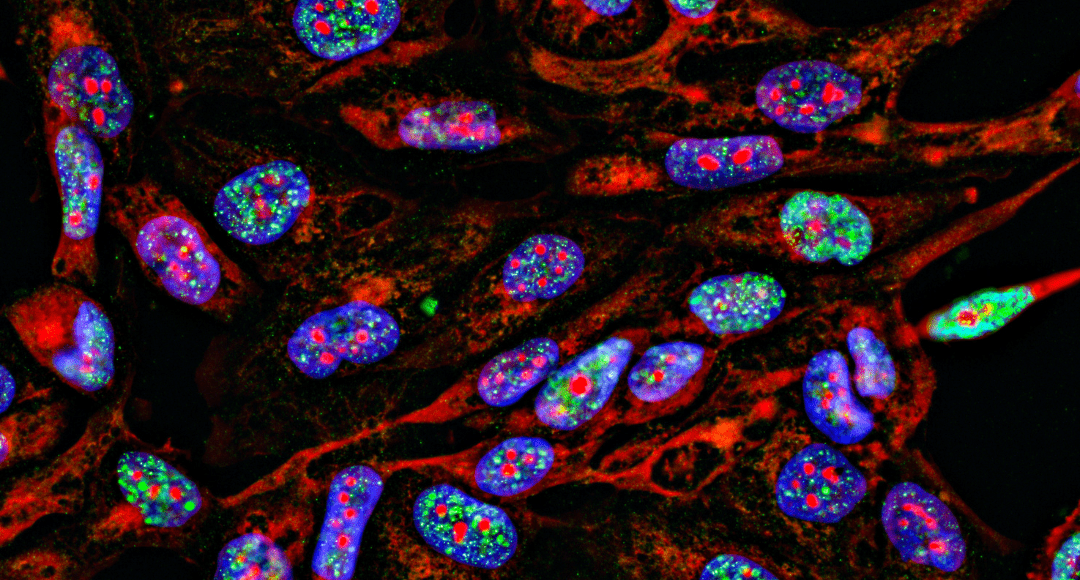
High-content imaging (HCI) combines automated fluorescence microscopy with advanced image analysis to study cellular samples. This article looks at the key steps in a typical HCI workflow and explores some common applications. It also highlights some of the leading solutions available for HCI-based research, with insights from Andy Bashford, Imaging Product Marketing Manager at Molecular Devices, and Stephen Full, Senior Product Manager for Automated Microscopy Systems at Thermo Fisher Scientific.
What is High-Content Imaging?
When discussing high-content imaging, it is important to understand how it differs from three other interconnected practices: high-throughput screening (HTS), high-content screening (HCS), and high-content analysis (HCA). High-throughput screening is the process of testing high volumes of samples, such as large compound libraries or genetic mutations, for a specific biological response. Screening assays can be cellular or biochemical in nature (e.g., viability, marker expression, enzyme activity, binding interactions, reporter gene activity) and typically use miniaturization with automation to facilitate throughput.
High-content screening was developed to complement HTS, driven by the pharmaceutical industry’s need for a platform that could yield better, functional information in the discovery process1. The first HCS platform, the ArrayScan®, was launched in 1997 by Cellomics, Inc. (now part of Thermo Fisher Scientific). This automated, cell-based imaging system enabled researchers to study compound effects on cell morphology, protein localization, and more by detecting multiple markers simultaneously with immunofluorescence1,2. In recent years, advanced technologies including confocal optics, water immersion objectives, and AI-analysis tools have enhanced the capabilities of HCS systems to improve performance and enable a wider range of applications.
The terms high-content imaging and high-content analysis were coined to describe the capture and analysis of any cell image data, irrespective of whether it involved using an HCS platform. A defining feature of HCA is its use of sophisticated algorithms for quantifying cellular features from the acquired images, which can help link complex cellular phenotypes to in vivo behaviors.
The High-Content Imaging Workflow
The HCI workflow begins with preparing samples. Traditionally, these were fixed 2-dimensional (2D) cell cultures in microplates, but HCI is increasingly being applied to study live cells, 3D cultures, and tissue sections. To allow for visualizing specific cellular components or biomarkers of interest, the samples are incubated with fluorescent probes, such as nuclear stains or fluorescently-labeled antibodies. The microplates are then loaded into an automated microscope, often with the aid of a robotic plate handler to increase assay throughput. Following image acquisition, advanced HCA software is used to extract a wide range of quantitative measurements. These encompass cellular morphology, as well as protein expression levels and subcellular localization, and can reveal how cells respond to different treatments or stimuli.
High-Content Imaging Applications
Besides being used to augment high-throughput screening campaigns by providing functional information on cells, HCI supports many other applications. These include cellular pathway analysis, which can help determine the mechanisms underlying disease, and characterization of stem cells, which is important for regenerative medicine applications. Other HCI applications include assessing the efficiency and effects of gene delivery into cells, investigating neurite outgrowth, and studying the dynamics of viral infection. HCI is also used for toxicology studies based on measurements of cellular viability, apoptosis, or oxidative stress, which can help identify compounds that may pose a risk to human health.
Leading Solutions for High-Content Imaging
High-content imaging technologies vary in terms of the number of markers they can simultaneously detect, the level of throughput they can provide, and their ease of use. The following are some of the leading solutions:
ImageXpress® HCI.ai High-Content Screening System (Molecular Devices)
In January 2025, Molecular Devices launched their next generation imager, the ImageXpress HCS.ai High-Content Screening System. The platform is based on state-of-the-art AgileOptix™ technology, which combines proprietary, dual-spinning disk technology, an advanced solid-state light engine, and a sensitive scientific CMOS sensor to enable exceptional imaging of cells and subcellular components. “The all new MetaXpress Acquire software streamlines complex workflows, minimizing training times and helping the whole lab get the most out of the system,” reports Bashford. “AI-powered IN Carta® image analysis software leverages cutting edge tools to improve the accuracy and robustness of high-content image analysis.”
The ImageXpress HCS.ai offers multiple imaging modes: brightfield label-free imaging, widefield, and confocal fluorescent imaging. It also has a high-intensity laser light source option, enabling researchers to capture images faster with shorter exposure times and multiplex their experiments with 7 lasers and 8 imaging channels. Other noteworthy features of the ImageXpress HCS.ai include fully automated water immersion objective lenses and a magnification changer, which provides up to 12 magnifications in a single configuration. The system also offers a Deep Tissue Confocal Disk, designed to improve data quality from thick 3D samples, as well as Environmental Control for live cell experiments. “The flexible, modular design allows users to choose the right configuration and upgrade it in the future, letting them invest in a quality system that can grow as their needs develop,” says Bashford.”
Figure 1. ImageXPress HCS.ai High-Content Screening System.
ImageXpress® Pico Automated Cell Imaging System (Molecular Devices)
The ImageXpress Pico Automated Cell Imaging System offers researchers an accessible solution to high content imaging, combining high-resolution imaging with powerful analysis. The imager can run fluorescent and brightfield assays with 2.5X – 63X magnification, 6 filter positions, Z-stacking, Autofocus, Live Preview, and optional features such as Environmental Control and Digital Confocal 2D on-the-fly deconvolution, helping researchers to make the most out of their samples. “The system comes with over 25 pre-configured templates, ranging from simple cell counting to sophisticated neurite tracing,” reports Bashford. “The user-friendly, icon driven software makes it easier than ever to get started with high content imaging.”
CellInsight™ High-Content Screening Platforms (Thermo Fisher Scientific)
Thermo Fisher Scientific’s CellInsight High-Content Screening Platforms include the CellInsight CX5, which lets researchers analyze cellular samples in up to five fluorescent colors; the CellInsight CX7 LED Pro, which enables 7-color detection and is well-suited to time-lapse and live-cell imaging studies; and the CellInsight CX7 LZR Pro, which provides high throughput and supports 3D organoid and spheroid research involving thick specimens. “All of our CellInsight High-Content Screening Platforms offer high-speed image acquisition and powerful image processing algorithms, letting users parallelize image capture and analysis for multiplexed cytometry measurements in real time,” says Full. “To help researchers decide which platform best meets their needs, we’ve compiled key features, specifications, videos, and sample data on our website. We also suggest compatible reagents and assays for monitoring cell viability, proliferation, and function, or determining cell structure.”
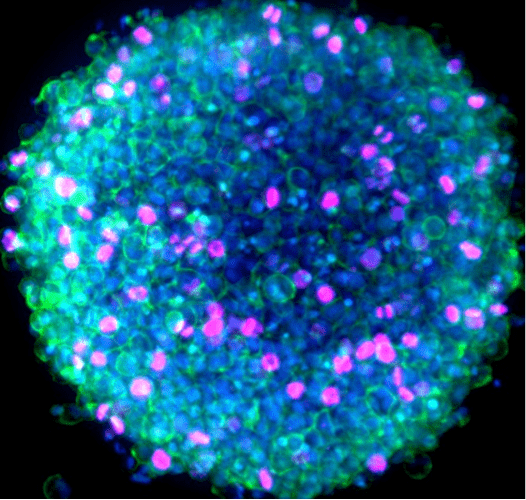
Figure 2. Immunofluorescence image of a HeLa spheroid captured with the CellInsight CX7 LZR Pro. The sample was labeled with Ki67 antibody followed by Invitrogen Alexa Fluor 647 and Alexa Fluor 488 phalloidin and Hoechst 34580 stains for nuclei. Imaging was performed using a 10x objective and 70 micron pinhole setting.
EVOS Cell Imaging Systems (Thermo Fisher Scientific)
EVOS Cell Imaging Systems are designed to make high-end microscopy simple, supporting applications from basic cell culture through to advanced multiplex tissue imaging. “All of our EVOS microscopes can be self-installed and running within an hour,” comments Full. “Users have the option of using our basic instrument, the EVOS M3000 Imaging System, which is capable of two-color fluorescence and can automatically measure cell confluency in real time. Alternatively, for more demanding cell-based imaging applications, we offer the semi-automated EVOS M5000 Imaging System and the fully automated EVOS M7000 Imaging System. For researchers working within the spatial biology field, the EVOS S1000 Spatial Imaging System features spectral unmixing software that enables a single round of 9-plex multiplex immunofluorescence to be imaged faster than instruments utilizing cyclic technology.” To support applications involving high-content screening or analysis, Thermo Fisher Scientific has developed the EVOS M7000 HCA Package, which combines the M7000 instrument with Celleste 6 Image Analysis Software to simplify the interpretation of results.
Supporting Your Research
FluoroFinder has developed a suite of tools that can help streamline the design of your high-content imaging experiment. Use our Antibody Search function to find antibodies that are validated for immunofluorescence, then leverage our Spectra Viewer to confirm which dyes are compatible with your imaging system. For an in-depth look at the optical properties and spectral profiles of different dyes, check out our Fluorescent Dye Database for detailed information on more than a thousand different fluorochromes.
References:
- Taylor DL. A Personal Perspective on High-Content Screening (HCS): From the Beginning. Journal of Biomolecular Screening. 2010;15(7):720-725. doi:10.1177/1087057110374995
- Way GP, Sailem H, Shave S, et al. Evolution and impact of high content imaging. SLAS Discov. 2023;28(7):292-305. doi:1016/j.slasd.2023.08.009