When using established methods for immunofluorescent staining of tissue sections, the need to avoid spectral overlap limits the number of markers that can be detected. This can mean that important information is missed, which is of particular significance when working with samples from rare donors. By allowing for more markers to be measured per sample, cyclical immunofluorescence promises deeper insights that can accelerate future research. Here, Danielle Fails, M.Sc., Spatial Biology Manager at Bethyl Laboratories (a Fortis Life Sciences® company) comments on some of the cyclical immunofluorescence methods available to researchers and explains how Bethyl Laboratories’ antibodies are being used in sequential immunofluorescence (seqIF™) on Lunaphore’s COMET™ platform.
What is Cyclical Immunofluorescence?
During traditional immunofluorescent staining of tissue sections, markers of interest are detected with either fluorescently labeled primary antibodies (direct detection) or a combination of unlabeled primary antibodies and fluorescently labeled secondary antibodies (indirect detection) before being imaged with a fluorescence microscope. However, because most fluorescence microscopes have only a handful of lasers and detectors, researchers can typically measure just 2 – 4 markers before spectral overlap prohibits the analysis of further targets. Cyclical immunofluorescence overcomes this constraint by using successive rounds of staining. As well as allowing for the same fluorophores to be re-used, this can both save time and improve experimental reproducibility by incorporating automation.
Cyclical Immunofluorescence Methods
A growing number of cyclical immunofluorescence methods is available to researchers. We recently featured CellScape™ Technology from Canopy Biosciences (a Bruker company) in our spotlight on Multiparameter Fluorescent Imaging, but here are some additional options:
-
Tyramide Signal Amplification (TSA)
TSA is one of the best-known forms of cyclical immunofluorescence. It involves incubating the sample (either cryopreserved or FFPE tissue) with unlabeled primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies before adding the TSA substrate, which is oxidized by HRP to deposit a tyramide-fluorophore at the target site. The antibodies are then removed with heat-induced epitope retrieval (HIER) and the staining process is repeated using a different fluorophore for each additional target.
“Advantages of TSA include its simplicity and capacity for signal amplification, which can improve the detection of low abundance targets,” reports Fails. “However, the order in which the different antibodies are added must be carefully optimized since repeated HIER can alter the antibody/epitope interaction.” At Bethyl Laboratories, TSA is performed using Opal™ reagents for detecting up to 6 targets plus DAPI with a PhenoImager™ HT (Akoya Biosciences), but TSA is compatible with many other reagents and imaging systems.
To discover which antibodies Bethyl Laboratories has validated and order tested on the PhenoImager™ HT, visit fortislife.com/primary-antibodies/phenoimager
-
Tissue-based Cyclic Immunofluorescence (t-CyCIF)
t-CyCIF evolved from cyclic-IF, a method first reported in 2015 for high multiplicity single-cell staining1. During t-CyCIF, FFPE tissue sections are stained with fluorescently labeled antibodies for three different protein antigens, plus a DNA dye, prior to image capture with a fluorescence microscope. The fluorophores are then bleached in a high pH hydrogen peroxide solution in the presence of light before another round of immunostaining is performed. t-CyCIF permits one cycle of indirect detection, prior to repeated cycles of direct detection, and allows for measuring up to 60 different markers2.
-
Co-Detection by Indexing (CODEX)
CODEX, developed in 2018, uses DNA-barcoded antibodies for detecting proteins in either cryopreserved or FFPE tissue sections3. All of the tagged antibodies are added to the sample at once, then pairs of antibodies are sequentially visualized using fluorescent dNTP analogs. Following image capture, the fluorophores are cleaved and washed away, and the next cycle is initiated. In the original publication, detection of as many as 66 different antigens was reported, although the authors suggest that this could be increased by adapting the method to include an ‘activation primer’.
-
Iterative Indirect Immunofluorescence Imaging (4i)
4i is a method that uses an oxygen radical scavenging buffer during fluorescence imaging to prevent any generated oxygen radicals from crosslinking antibodies to their bound epitopes in FFPE tissue sections4. The lack of crosslinking allows for antibody removal using relatively mild conditions and minimizes the risk of residual signal being carried over from previous staining cycles. A protocol has recently been published that permits staining of up to ~80 unique epitopes using off-the-shelf primary and secondary antibodies and commonly available chemical reagents5.
-
Sequential Immunofluorescence (seqIF™)
seqIF™ uses off-the-shelf, non-conjugated primary antibodies or fluorescently labeled primary antibodies for detecting targets of interest in cryopreserved or FFPE tissue sections6. It involves repeated sequences of staining, imaging, and elution of antibody complexes, and has been implemented on the COMET™, a fully automated platform based on a unique microfluidic chip technology.
A major advantage of COMET™ seqIF™ is that gentle, elution-based signal removal, coupled with short incubation times of just 2-4 minutes, preserves tissue antigenicity and morphology over multiple staining cycles. Consequently, the same slide can be used for additional downstream modalities, such as H&E, without the need for tissue transfer steps or additional serial tissue sections. Furthermore, because COMET™ seqIF™ works with standard, unlabeled primary antibodies, complex upstream conjugations can be avoided to eliminate undesired variability.
Using Bethyl Laboratories’ antibodies for COMET™ seqIF™
To date, Bethyl Laboratories has validated over 100 of its antibodies for use on the COMET™, with many more due for testing. An up-to-date list of these products can be found at fortislife.com/primary-antibodies/comet. “In our labs, we use the Epredia PT Module to standardize pretreatment before performing COMET™ seqIF™,” reports Fails. “Once the immunostaining process is complete, we use COMET’s integrated Lunaphore HORIZON™ image analysis software for data analysis. Because this approach removes a lot of the manual handling associated with traditional immunofluorescent staining it frees up researchers’ time to be spent on other tasks, as well as helps to improve experimental reproducibility.”
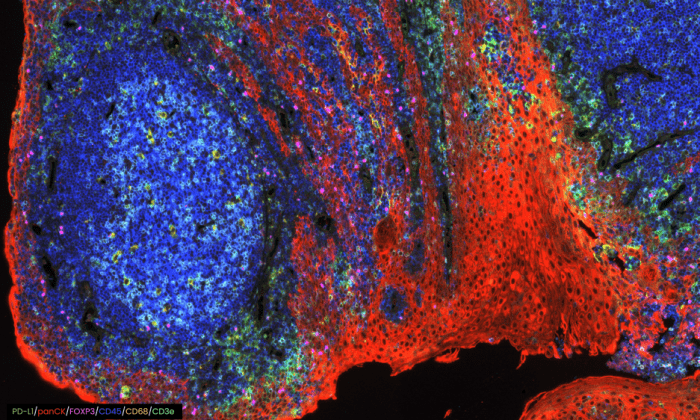
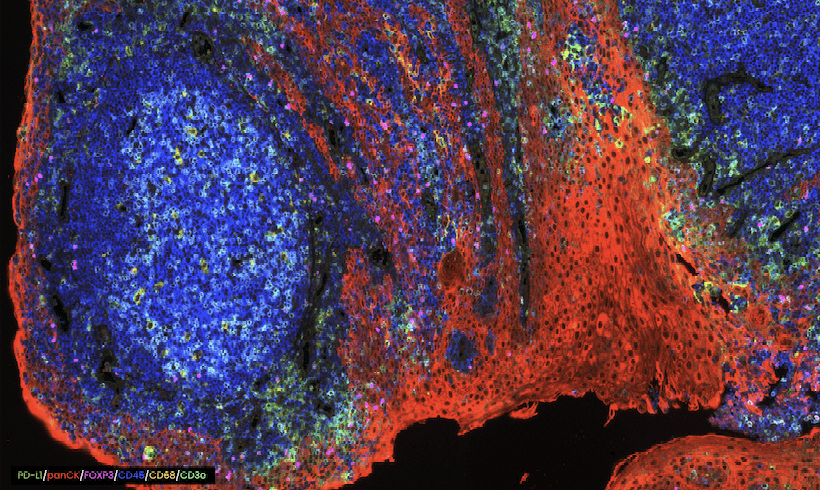
COMET™ seqIF™ staining of whole tonsil with antibodies from Bethyl Laboratories.
Supporting Your Research
FluoroFinder has developed a suite of tools to help you design and execute your experiments with ease. Use our Antibody Search function to find antibodies that have been validated for your chosen application and our Spectra Viewer to compare the spectral properties of more than 1,000 dyes alongside instrument-specific laser and filter configurations.
And don’t forget that our partners offer a wealth of resources to streamline your research. Check out Bethyl Laboratories’ Spatial Biology page to learn more about TSA and how Bethyl Laboratories researchers are using COMET™ seqIF™. Visit Lunaphore’s website for more information about the universal COMET™ technology, including its fully-automated, same-section hyperplex multiomics application, and a Tech Note demonstrating the high antibody elution efficiency, intact tissue morphology, and epitope stability achieved with the COMET™ platform.