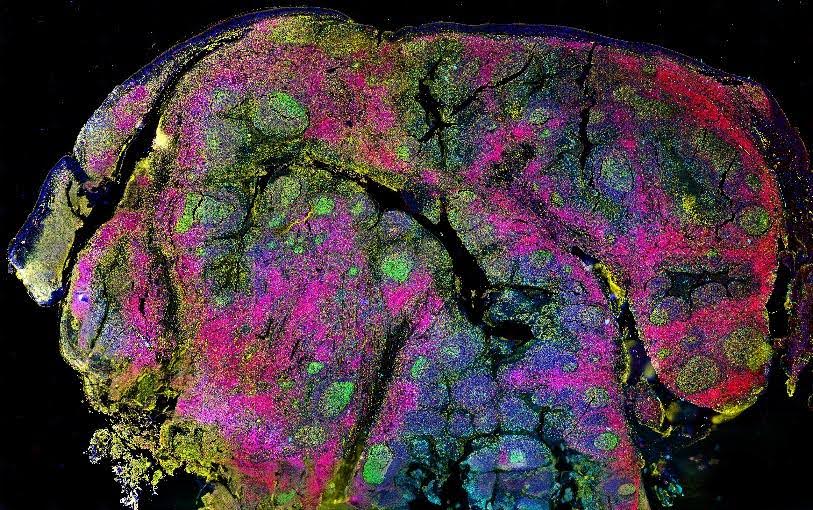
Multiparameter fluorescence imaging offers many advantages for scientific research. Not only does it use significantly less sample than generating a series of single-analyte images, but it also saves time and can provide a more complete picture of the system in question. Over the past few decades, various tools and technologies have been developed that address known challenges for multiparameter fluorescence imaging. We spoke with Tim Sindelar, Product Manager for Spatial Biology at Canopy Biosciences (a Bruker company), and Kristie Krug, CEO and COO at Core Quantum Technologies, to learn more about CellScape™ and MultiDots – two of the innovative solutions available to support multiparameter fluorescence imaging studies.
Common Challenges for Established Multiparameter Fluorescence Imaging Methods
The most established techniques for multiparameter fluorescence imaging are immunohistochemistry (IHC), which uses tissue sections, and immunocytochemistry (ICC), which generally uses adherent cells. Although these two methods are distinct, they share the same main protocol steps, specifically blocking, immunostaining, counterstaining, mounting, and imaging.
When performing these types of experiments, researchers have historically been limited to detecting just a handful of targets. This is to avoid spectral overlap, which occurs when a fluorescent signal spills over into a detector channel where it is not expected to show up and can be incorrectly interpreted. Other challenges for multiparameter fluorescence imaging stem from photobleaching, which is especially problematic for live-cell imaging studies that take place over an extended period of time, and the need to identify fluorophores with sufficient brightness for detecting low abundance targets. Fortunately, companies such as Canopy Biosciences and Core Quantum Technologies have found ways of addressing these issues.
Increased Multiplexing Capabilities with CellScape
According to Sindelar, Canopy Biosciences’ CellScape platform lets researchers measure a virtually unlimited number of protein biomarkers in a single sample. “CellScape technology is based on iterative cycles of staining, imaging, and photobleaching,” he explains. “Researchers add up to five different fluorescently-labeled primary antibodies, from any trusted supplier, at a time, then capture a high-resolution image before exposing the sample to UV light. Repetitions of this cycle yield a composite image that allows for algorithm-based population analysis with open-access software such as QuPath and ImageJ, as well as popular paid software such as Visiopharm and Enable Medicine.”
When asked whether steric hindrance from a build-up of antibodies on the sample can create problems, Sindelar notes that this is rarely the case. “When validating our off-the-shelf modular antibody panels, we run each assay front-to-back and then back-to-front to ensure there are no changes in signal intensity that would suggest steric hindrance,” he says. “We also test every antibody in singleplex to confirm that we see the expected staining pattern.”
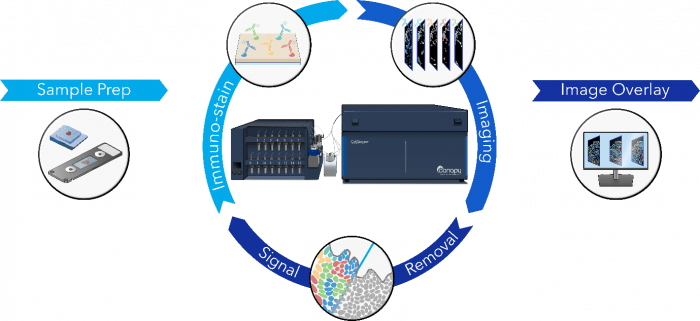
Figure 1. The CellScape imaging process.
CellScape technology was originally developed to improve on flow cytometry, which it achieves in several ways. “First, because CellScape allows researchers to visualize individual cells, which flow does not, it provides both quantitative and qualitative results to increase confidence in the data,” says Sindelar. “Second, CellScape simplifies high-plex assay design by enabling an assay to be broken down into spectrally distinct 5-plex cycles. Third, CellScape allows for data-driven assay expansion – once a tissue has been stained with a biomarker assay, and the resulting data queried, the same tissue section can be stained with a different biomarker assay as influenced by prior findings.”
Importantly, these benefits translate to improvements on traditional IHC, where CellScape additionally removes operator bias from phenotyping. “Traditional IHC is a subjective assay, with phenotyping based on scores as decided by the human eye,” says Sindelar. “In contrast, CellScape employs sophisticated algorithms to automatically segment single cells, and query the fluorescent intensity of each biomarker within each cell to classify cells into phenotypes, while retaining their mapped position within the tissue.”
Other advantages of CellScape include an 8-log high dynamic range (HDR), which allows for detecting both scarce and abundant targets, and a large imaging window. “We recently launched a Whole Slide Imaging Chamber that lets us see more tissue than anyone else in spatial proteomics,” notes Sindelar.
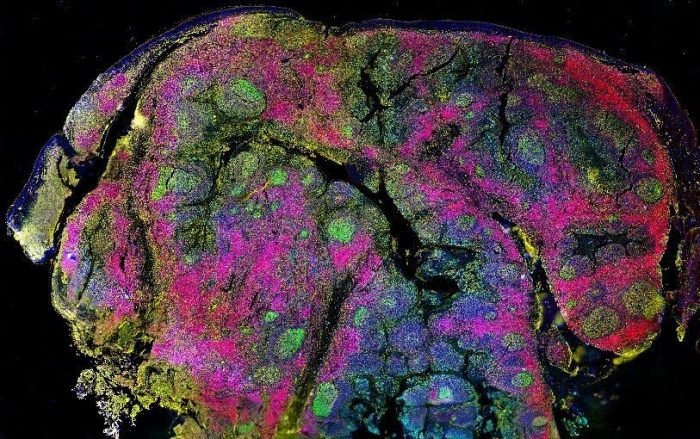
Figure 2. CellScape HDR biomarker capture in human tonsil.
Bright, Tight, and Highly Photostable Emission with Quantum Dots
Quantum dots are semiconductor nanocrystals whose properties are determined by their physical size. Their uses span television and computer screen technology through to precision guidance for the surgical removal of cancerous tissue, and their discovery and synthesis saw Moungi G. Bawendi, Louis E. Brus, and Alexei I. Ekimov awarded the 2023 Nobel Prize in Chemistry.
“In a research setting, quantum dots can offer several advantages over traditional fluorescent dyes,” reports Krug. “First, the unique properties of quantum dots allow for multiple probes to be excited with the same laser, which simplifies experimental set-up. Quantum dots are also much brighter than fluorescent dyes, with a narrower emission bandwidth, meaning they have better detection capability and less signal overlap. Additionally, quantum dots do not photobleach, which makes them particularly useful for live-cell imaging studies.”
To enable researchers to leverage these benefits, Core Quantum Technologies has developed a proprietary technology for encapsulating quantum dots in a protective micelle polymer. The resultant MultiDots have a hydrophilic portion with functional groups that allow for antibody attachment, and a hydrophobic portion that serves to protect the quantum dot fluorescence, as shown in Figure 3.
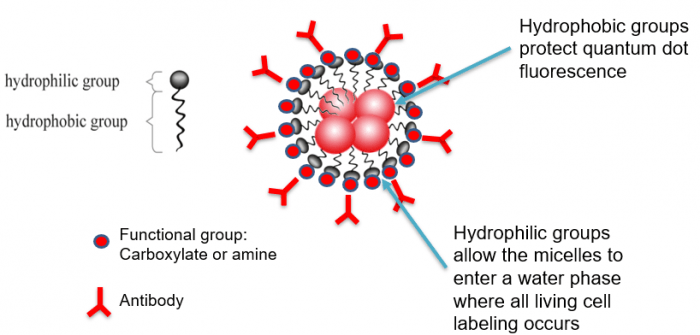
Figure 3. MultiDots (polymer-protected quantum dots) from Core Quantum Technologies.
When using MultiDots for multiparameter fluorescence imaging, optimal excitation requires either a UV (350 nm) or Violet (405 nm) laser, while emission wavelengths span 420 to 800 nm, with FWHM values ranging from just 21 to <80 nm. “By providing sensitive, photostable signal output and the ability to add emission every 20 nm, MultiDots increase flexibility for experimental design,” says Krug. “Ultimately, this means more time analyzing and less time setting up, which can help to reveal novel insights.”
Supporting Your Research
Whatever the aim of your multiparameter fluorescence imaging experiment, FluoroFinder is here to help. Use our Antibody Search tool to quickly find antibodies for your chosen target, species, and application. Then turn to our Spectra Viewer to identify the right fluorophores for your instrument’s laser and filter configurations. And remember to check out our partner’s websites for more information about CellScape and MultiDots, including how they can accelerate your research.