It has been almost half a century since Georges Köhler and Cesar Milstein first described hybridoma technology, which is still widely used for producing monoclonal antibodies today. Over the years, the methodology has evolved and alternative approaches have been developed to address limitations of the original technique. Here, Sam Sugerman, Ph.D., Principal Scientist, VHH Discovery at Fortis Life Sciences® and Miranda Lewis, Ph.D., Marketing Manager at Jackson ImmunoResearch discuss advances within the field.
Understanding Traditional Hybridoma Technology
Traditional hybridoma technology, as reported by Köhler and Milstein in 1975, begins by administering a protein antigen to a mouse via a series of injections over a period of several weeks1. This stimulates B cell differentiation into plasma B cells and memory B cells, of which the plasma B cells secrete antibodies into the bloodstream. Once a suitable antibody titer is detected in the serum, typically by ELISA, the mouse is sacrificed and the spleen removed to isolate the activated B cells using density gradient centrifugation.
Next, the B cells are fused with immortal myeloma cells using polyethylene glycol (PEG) and are cultured in a selective media for 10-14 days, such that only the hybrid cells survive. These so-called hybridoma are then transferred to microwell plates using the limiting dilution method, where they are allowed to proliferate, and the culture supernatants are screened to identify antibody-producing clones. Once favorable clones have been identified, they are expanded in vitro to allow for isolating the monoclonal antibodies from the media.
A main advantage of traditional hybridoma technology is that it provides an unlimited supply of highly specific and reproducible antibodies. However, the method is time-consuming and labor-intensive, usually taking 6 to 9 months, and the efficiency of cell fusion is low, typically around just 1%. In addition, traditional hybridoma technology is unsuitable for producing antibodies against small peptides and fragment antigens, and requires the use of animals – something that the EU Reference Laboratory for Alternatives to Animal Testing (EURL-ECVAM) and other organizations suggest should be reduced2.
Advances to Traditional Hybridoma Technology
The limitations of traditional hybridoma technology have led to various modifications being made to the original technique. Included among these, B cell targeting (BCT), also known as the pulsed electric field method, was described by Lo et al. in 1984 as a means of improving the efficiency of cell fusion3. During BCT, an avidin-conjugated antigen is captured by the surface immunoglobulins on B cells and subsequently binds to biotinylated myeloma; the application of an electric field then produces selective fusion of the cells that are in contact.
Another method, known as stereospecific targeting (SST), was developed by Tomita et al. in 2007 to produce better-targeted antibodies4. It includes introducing the antigen intramuscularly, in the form of DNA, to promote its native expression, and fusing the cells as described for the BCT method. As well as circumventing the need to express and purify protein antigens in large quantities, SST allows for obtaining antibodies against several proteins at the same time (through immunization with multiple DNA sequences) and enables targeting of complex or non-conventional antigens.
Other advances to traditional hybridoma technology include the use of fluorescence-activated cell sorting (FACS) to isolate individual hybridoma, which is more efficient than limiting dilution, and the replacement of PEG fusion with less cytotoxic techniques, such as the pearly chain method4.
Alternative Antibody Production Technologies
Of the various methods that have been developed as alternatives to traditional hybridoma technology, antibody phage display is the most established. A typical antibody phage display workflow involves immobilizing the antigen on a solid support, such as a microwell plate or a streptavidin-coated bead, before adding a library comprising tens of millions of phage, each expressing a different antibody binding domain at its surface. This is usually in the form of a single-chain fragment variable (scFv) region, a fragment antigen-binding region (Fab), or the variable domain of a heavy chain only antibody (VHH, see below for further detail). Following incubation and a wash step, the bound phage are eluted for amplification in bacteria and the cycle is repeated. By decreasing the antigen concentration each time, it is possible to identify phages displaying high affinity antibody fragments, which can be isolated for sequencing and expression, as well as be used in any downstream engineering workflows.
Depending on the source of the antibody variable (V) genes used for library generation, antibody phage display libraries are broadly classified as naïve, immune, synthetic or semi-synthetic. “Naïve antibody libraries are generated using V genes from non-immunized donors,” explains Sugerman. “As a result, they allow for identifying antibodies against a wide range of molecules. Although most of these antibodies will have relatively low affinities, owing to the fact that in vivo affinity maturation has not taken place, a major advantage of naïve libraries is that they let the end user cast a wide net for antibody discovery.”
Immune antibody libraries are instead constructed using V genes from immunized donors, meaning they are more focused and have higher affinities than naïve antibody libraries. “Advantages of both naïve and immune animal libraries are that they exhibit less genetic redundancy using typical techniques (e.g., saturation mutagenesis) as compared with synthetic or semi-synthetic libraries and every protein sequence has already been shown to express in mammalian systems,” notes Sugerman.
Synthetic antibody libraries are generated using artificially designed DNA, which eliminates the need for animal use and provides opportunities to generate antibodies targeting toxic antigens, such as snake venom5. “Synthetic libraries also make it easier to obtain large numbers of mutants and allow for removing liabilities like cysteine and lysine,” says Sugerman. Semi-synthetic antibody libraries are produced using both naturally and synthetically randomized complementary determining regions (CDRs).
Other display methods include yeast surface display and mammalian surface display, as well as mRNA display and ribosome display.
Single B cell antibody technologies represent another means of antibody production, which is based on isolating individual B cells from blood or lymphoid tissue samples and cloning the antibody genes. Importantly, these methods circumvent the time-consuming process of immortalization by either 1) using optimized culture conditions to maintain the antibody-secreting B cells throughout antibody screening, or 2) enriching the antigen-specific B cells to minimize culture times6. As well as enabling rapid antibody production, single B cell antibody technologies allow for using human samples. However, these benefits are offset by technical complexity and high costs.
Other Antibody Hosts
Although traditional hybridoma technology was developed using mice, the methodology has been extended to encompass rabbits and rats. Researchers may also use non-hybridoma-derived antibodies from many other host species. “Camelid antibodies have become especially popular in recent years as their smaller size compared to conventional IgG antibodies, combined with their tractability for recombinant engineering, makes them useful as both therapeutics and as research tools for imaging,” reports Lewis. “In a therapeutic setting, the enhanced tissue penetration, solubility, and thermal stability of recombinant VHH format antibodies are especially advantageous. Whereas when performing imaging and detection, polyclonal VHH antibodies can improve resolution by reducing the linkage error – an imaging artefact that results from the distance between the target antigen and the fluorophore due to the size of the antibody complex on which the fluorescent probe is conjugated.”
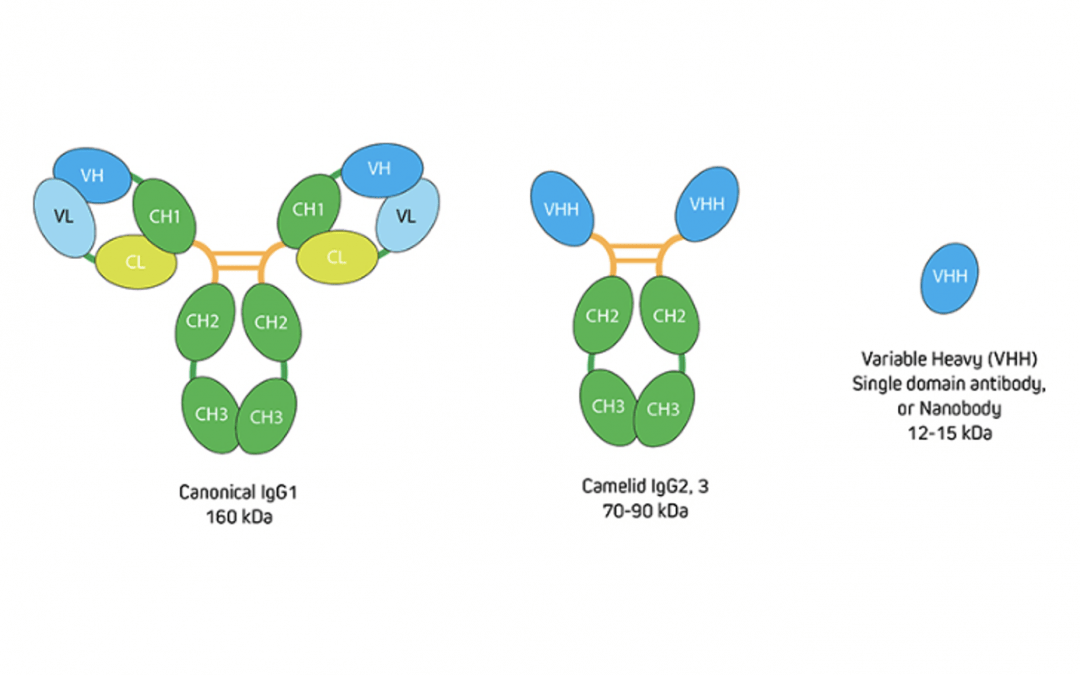
To put this into context, recall that IgG antibodies consist of two identical heavy chains and two identical light chains, giving them a molecular weight of approximately 150 kDa. Camelid antibodies instead have only heavy chains, from which an antigen-binding variable domain (VHH) fragment antibody can be generated that has a molecular weight of just 15 kDa (Figure 1). “If you have an antigen that is labeled indirectly with two conventional IgGs, the fluorescent signal could be over 300 kDa away from the target,” explains Lewis. “This can be problematic when you need to achieve nanometer resolutions for imaging, or when you want to perform FRET experiments where proximity to proteins is important. Choosing an indirect method that incorporates a VHH either as the primary or secondary antibody decreases the size of that complex (Figure 2) to reduce the distance between the probe and target antigen – which can make a big difference to the achievable resolution. And, by selecting polyclonal antibody reagents, researchers can benefit from signal amplification.”
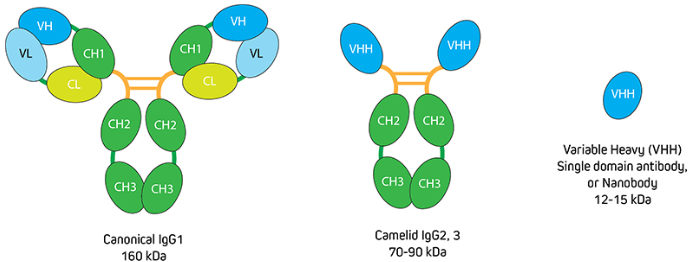
Figure 1. Comparison of conventional IgG with camelid immunoglobulins and VHH. Image provided by Jackson ImmunoResearch.
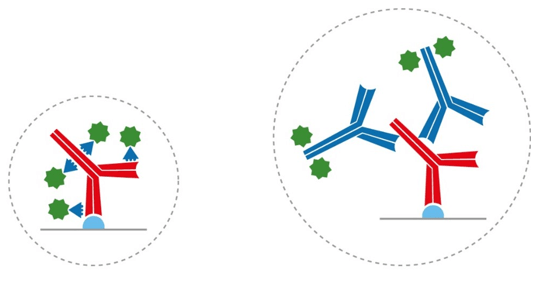
Figure 2. Indirect detection with a conventional IgG and a labeled VHH (left), and with two conventional IgG antibodies (right). Using VHH antibodies can reduce linkage error.
Other host species seeing increased interest from researchers include sharks, which also produce antibodies with only heavy chains that have a very small antigen-binding variable domain, and lamprey, which use variable lymphocyte receptors (VLRs) for antigen recognition. VLRs have a molecular weight of approximately 35 kDa and, due to their phylogenetic distance from man, are of particular interest for therapeutic applications, such as blood-brain-barrier (BBB) targeting7. A more familiar species, the chicken, also exhibits an evolutionary difference from man, which has long been exploited for signal amplification or to avoid interference in immunological assays8.
Supporting Your Research
With so many antibodies now available, choosing the right product may seem overwhelming. Fortunately, FluoroFinder is here to help with our suite of tools to simplify the design of your experiment. Use our Antibody Search function to find antibodies that have been validated for your chosen application, which lets you filter on host species, clonality, and other key features. And, for fluorescence-based research, use our Spectra Viewer to compare the spectral properties of more than 1,000 dyes alongside instrument-specific laser and filter configurations.
In addition, our partners offer an extensive range of high-quality products and resources. Jackson ImmunoResearch has developed polyclonal AffiniPure-VHH™ Secondary Antibodies against a range of species, which are cross-adsorbed and can be used for multiple labeling techniques, and polyclonal Whole IgG anti-VHH and Fab fragment anti-VHH secondary antibodies, which allow for detecting VHH antibodies that cannot be directly labeled. Fortis Life Sciences’ product offerings include a wide selection of traditional and recombinant monoclonal antibodies, as well as its AbNano™ VHH Naïve Library, a fully natural, single domain library constructed from 103 naïve camelids (77 llamas and 26 alpacas) that has been shown to provide nanomolar VHH binders of PD-L1 and other therapeutic targets.
References
- https://pubmed.ncbi.nlm.nih.gov/1172191/
- https://publications.jrc.ec.europa.eu/repository/handle/JRC120199
- https://pubmed.ncbi.nlm.nih.gov/6088990/
- https://pubmed.ncbi.nlm.nih.gov/35492397/
- https://pubmed.ncbi.nlm.nih.gov/29890762/
- https://pubmed.ncbi.nlm.nih.gov/34743921/
- https://pubmed.ncbi.nlm.nih.gov/35411012/
- https://pubmed.ncbi.nlm.nih.gov/10680951/