When selecting reagents for flow cytometry, fluorescently-labeled antibodies are likely the first products that come to mind. However, supplementary reagents are also important and it pays to investigate what’s on offer. This article looks at some of the different types of supplementary reagents you should consider for your flow cytometry experiment, with insight from Mike Blundell, Ph.D., Product Manager at Bio-Rad, Eric Torres, Ph.D., Marketing Manager at Biotium, and Christopher Manning, Associate Director, Flow Cytometry at Cell Signaling Technology (CST®).
Cell Viability Dyes
Exclusion of dead cells in flow cytometry is essential to prevent false positives. Dead cells have high autofluorescence and bind to antibodies non-specifically, which can lead to inaccurate interpretation of results. One way of measuring cell viability is to use non-permeant DNA binding dyes, such as propidium iodide (PI), DAPI, or 7-AAD, which can only enter cells with compromised membranes. Biotium’s NucSpot® Nuclear Stains are another option, giving researchers access to a wider selection of colors.
Alternatively, if an experiment requires fixed cells, a fixable cell viability dye should be chosen. “Fixable cell viability dyes typically work by binding free amines on the cell surface, as well as intracellular amines that are exposed in cells with compromised cell membranes, meaning that dead or dying cells having a greater level of fluorescence than their viable counterparts,” explains Blundell. Available options include Bio-Rad’s VivaFix Dyes, Biotium’s Live-or-Dye™ Fixable Viability Stains, and Ghost Dyes from CST.
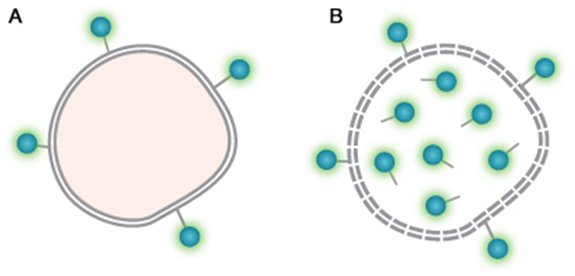
VivaFix Cell Viability Assay chemistry. A. Live cells with VivaFix Dye bound to surface primary amines. B. Dead cells with VivaFix Dye bound to surface and intracellular primary amines.
Activation Reagents
Activation reagents are used for purposes that include stimulating intracellular signaling pathways, increasing cytokine production, and driving cell proliferation. The type of activation reagent you choose will depend on the aim of your flow cytometry experiment. “At CST, we offer an extensive selection of Cytokines and Growth Factors, as well as a growing number of Small Molecule Compounds, to meet various cell activation needs,” reports Manning. “In addition, we have developed Rapid-Act T Cell Activation Kits for both human (#88179) and mouse (#86772), which enable easy, single-step induction of T cell activation and proliferation. We also provide a Cell Stimulation Cocktail, either with (#23318) or without (#29255) protein transport inhibitors, to increase cytokine production for detection by downstream immunoassays.”
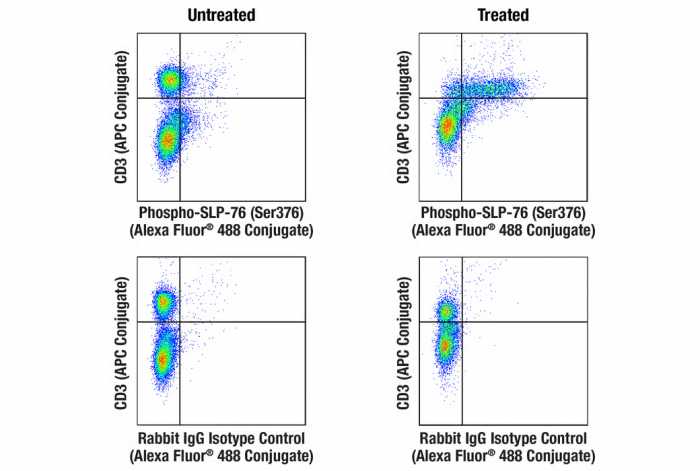
Rapid-Act T Cell Activation. Flow cytometric analysis of mouse splenocytes, untreated (left column) or treated with Rapid-Act T Cell Activation Kit (Mouse, Anti-CD3/CD28) (15 min; right column), using Phospho-SLP-76 (Ser376) (E3G9U) XP® Rabbit mAb (Alexa Fluor® 488 Conjugate) #47876 (top row) or concentration-matched Rabbit (DA1E) mAb IgG XP® Isotype Control (Alexa Fluor® 488 Conjugate) #2975 (bottom row), and co-stained with CD3 (17A2) Rat mAb (APC Conjugate) #24265.
Protein Transport Inhibitors
Protein transport inhibitors such as monensin and Brefeldin A are commonly used to enhance intracellular cytokine staining. They achieve this by preventing cytokine secretion, which leads to a rapid accumulation of cytokines in the Golgi apparatus and endoplasmic reticulum. “Both monensin and Brefeldin A are widely available, although the choice and culture conditions for these inhibitors may require optimizing for the species and cytokines being stained,” cautions Blundell.
Red Blood Cell Lysis Buffers
Red blood cell (RBC) lysis is routinely performed when working with whole blood or tissue homogenates in order to properly gate leukocytes. It can involve treating the entire sample prior to staining the remaining cells with fluorescently-labeled antibodies or can be performed once the staining process is complete. In either scenario, it is critical that the lysis buffer has minimal effect on the leukocytes. Commercially available products include Bio-Rad’s Erythrolyse Red Blood Cell Lysing Buffer, which contains a fixative reagent, and CST’s RBC Lysis Buffer(10X) #46232, which is fixative-free.
Cell Cycle Staining Dyes
Cell cycle analysis based on the quantitation of DNA was one of the earliest applications of flow cytometry, and fluorescent dyes that bind stoichiometrically to DNA, such as DAPI, PI, Hoechst 33342, 7-AAD, DRAQ5® and DRAQ7™, have all stood the test of time. More recently, alternatives to these products have been developed to increase flexibility for experimental design, including Biotium’s NucSpot® 470 and NucSpot® Far-Red Nuclear Stains. “NucSpot 470 enables selective detection of dead cells by flow cytometry in the FITC channel, while NucSpot Far-Red is an improved alternative to 7-AAD with a red-shifted fluorescence emission for less bleed-through in the PE-Texas Red® channel,” reports Torres.
Cell Proliferation Dyes
Tracking cell proliferation/division is common in flow cytometry. It has traditionally been performed using carboxyfluorescein succinimidyl ester (CFSE), an amine-reactive derivative of fluorescein that is non-fluorescent until it enters viable cells, where it is hydrolyzed by cytoplasmic esterases to yield green fluorescence. However, limitations of CFSE include cell toxicity, leakage from the cell, and bleed-through into the PE and PE-TexasRed® channels.
To address these issues, Biotium has developed ViaFluor® SE Cell Proliferation Dyes. “Our ViaFluor SE Cell Proliferation Dyes improve on many of the properties just described,” says Torres. “They include ViaFluor® 405 SE and ViaFluor® 650 SE, which are respectively detected in the Pacific Blue® and APC channels, and ViaFluor® 488 SE, which can directly replace CFSE for detection in the FITC channel. Not only do ViaFluor Dyes offer better peak separation than CFSE, but they also have less leakage and lower toxicity than CFSE when used at the recommended concentration.”
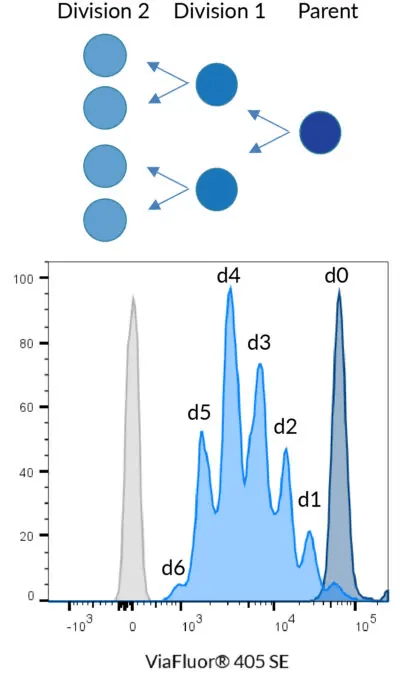
Principle of cell division tracking with ViaFluor® Cell Proliferation Dyes. When a stained cell divides, each daughter cell receives half the dye in the parent cell, with each cell division represented as a successively dimmer population on a flow cytometry histogram. Data shown using 5 µM ViaFluor® 405 to stain PBMCs.
Bio-Rad’s CytoTrack Cell Proliferation Dyes are another option, supplied in blue, green, and red emission spectra. “CytoTrack Dyes are compatible with standard formaldehyde-containing fixatives and saponin-based permeabilization buffers,” notes Blundell. “Compared to CFSE, they provide clearer resolution of up to ten cell generations.”
Fc Blocking Reagents
Fc receptors on the surface of immune cells such as monocytes, macrophages, and B cells bind antibodies via their constant (Fc) domains, which can lead to false positives. A common way of avoiding this problem is to introduce an Fc blocking step into the staining protocol, such that only antigen specific binding is observed. For this approach to be effective, the Fc blocking reagent should be matched to the sample host species. Available products include Bio-Rad’s Human Seroblock and Mouse Seroblock, and CST’s Human Fc Receptor Blocking Solution #58948 and Mouse Fc Receptor Blocking Solution (CD16/CD32) Rat mAb #88280.
Buffers
Flow cytometry experiments use many different types of buffers, including for fixation, permeabilization, and immunostaining. While many of these can be made in-house, researchers often prefer to use commercial products for the greater consistency they can provide.
“Besides standard flow cytometry buffers, CST offers several reagents that address specific research needs,” comments Manning. “For example, our FluoroClear Blocking Buffer #33449 is a fairly unique product that reduces fluorescent background resulting from weak fluorophore interactions in fixed/permeabilized cells without reducing specific signal resulting from antibody-target interactions. We also offer several flow cytometry fixation/permeabilization kits that enable researchers to exactly replicate the conditions that were used during our extensive antibody validation process. These include our Intracellular Flow Cytometry Kit (Methanol) #13593 and Intracellular Flow Cytometry Kit (Triton X-100) #51995. The inclusion of the permeabilization reagent in the product name is important so researchers can verify proper permeabilization for their targets of interest, and to avoid unintended problems, such as denaturation of protein fluorophores with methanol.”
Supporting Your Research
If you need help with selecting the right dyes to monitor cell viability, perform cell cycle analysis, or track cell proliferation, you can count on FluoroFinder! Use our Fluorescent Dye Database to explore the optical properties and spectral profiles of more than a thousand fluorochromes, and enter your instrument laser and filter combinations into our interactive Spectra Viewer to find the best fluorochrome for your application.