Structural biology is focused on mapping where proteins, lipids and other biological molecules are located in cells and tissues in space. Researchers often use immunofluorescence labelling techniques that have been around for decades to visualize biomolecules and their interactions. In summary, a primary antibody is used to recognize the molecule of interest (antigen). After washing away the excess, a fluorescently tagged secondary antibody binds to the primary antibody to provide the visual signal. It’s also common to use a fluorescently labelled primary antibody to simplify the process.
As researchers use more and more antibodies to label more and more antigens in a single sample, size and crowding become an issue, especially when attempting to visualize interactions between sub-cellular components. (Matos, 2020) Steric effects, or crowding of molecules in space, can be caused by antigens that are too close together for multiple antibodies to bind, or by epitopes that are hidden in complexes, etc. A typical IgG antibody is roughly 10 nm in diameter. This grows to 15 nm or more after the secondary antibodies are added. For reference, actin, a common structural protein, is about 6 nm in diameter, and green fluorescence protein (GFP) is a ~ 2.4 x 4.2 nm cylinder.
Another issue for structural biologists is that they often prefer to leave the samples as intact as possible, in whole mounts or thick sections, and diffusion of reagents into the interior can become a problem. Modern imaging methods, such as confocal, multiphoton, light sheet and the use of adaptive optics, enable researchers to look several millimeters into a specimen to better understand 3-dimensional relationships. It is important to have fluorescent labels that can quickly and deeply penetrate these thick tissue specimens. Unfortunately, antibodies, given their large size, have a slow diffusion constant through fixed tissue and, depending on the structure of the sample, may be too large to even reach the embedded antigens of interest.
Identifying Steric Effects in Multiplexed Immunofluorescence
The easiest way to identify steric effects in the sample is to compare images using a single-plex protocol with those of the multiplex protocol. A reduction of signal in the multiplexed case indicates that steric effects are playing a role. One can also change the labelling order of the multiplex protocol – changes in signal with labelling order are also an indication of steric effects. (Sayed, 2019) There are a few ways to reduce steric effects- reduce the size of the fluorescent label complex, increase the space between molecules of interest, or use sequential immunofluorescence labelling to bypass the problem.
For the case of diffusion problems in thick samples, a z-stack will reveal reduced or non-existent labelling in the center of the sample. Sometimes this can be improved by waiting (sometimes up to several days) for diffusion and labelling to occur throughout the sample.
There are three main approaches to steric and diffusion issues in highly multiplexed immunofluorescent samples. The size of the antibodies can be reduced, the sample itself can be expanded, and sequential staining of antigens can be used. These approaches are described in more detail below.
Approaches to Steric and Diffusion Issues
VHH Antibodies in Multiplexed Immunofluorescence
In 1993, a smaller antibody option was introduced in the form of the single-domain antibody (VHH antibodies). These began as VHH fragments isolated from camelid heavy-chain antibodies. Now, they are expressed and purified from both mammalian cells and E. coli in production volumes from a number of suppliers, including Fortis Life Sciences. Learn more about antibody production methods.
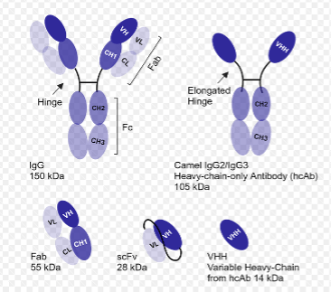
Figure 1: Size and composition of antibodies and antibody fragments (courtesy of Fortis Life Sciences)
VHH antibodies are on the order of 2-4 nm in diameter- roughly 1/10 the size of a typical IgG antibody (Figure 1) and about half of the size of GFP. They can be used in standard staining protocols as a replacement for both primary and secondary IgG antibodies. Because of their small size relative to mAbs and Fabs, there are slight affinity differences. According to Sam Sugarman, Director of VHH Discovery at Fortis Life Sciences, “VHH can get subnanomolar affinities, depending on the interaction of interest, but they’re going to be slightly weaker than corresponding mAbs or Fabs. This difference is more pronounced against small antigens or epitopes and less pronounced against complex folded proteins. VHH don’t typically get into low picomolar affinities or below without extensive engineering.”
For custom solutions, VHH antibodies can be a good option as Sam Sugarman explains “If the goal is to discover or mine a good sequence from existing sets, VHH is simpler to use in display systems; this means that good libraries are more accessible for teams with limited resources than a similar setup with Fab or full-length IgG might be. Fortis’s AbNano® VHH are naturally-derived from our herd of llamas and alpacas. This allows us access to framework sequences and permutations that would be inaccessible to teams using synthetic approaches or with limited numbers of animals. We have built our custom-discovery platform on more than a decade in the space, and we utilize a foundation of custom immunization approaches with the animals to generate unique clonotypes and molecules.”
VHH antibodies have been used to fluorescently immunolabel thick sections and even whole animals in a technique called vDISCO (VHH-boosted 3D Imaging of Solvent-Cleared Organs (vDISCO). (Cai, 2023) VHH antibodies are also being developed for clinical applications, particularly for cancer diagnosis and therapy. (Zhang, 2023) They have been shown to pass through the blood-brain barrier (Pothin, 2020), so they can be used for drug-delivery and photodynamic therapy applications throughout the body.
Expansion Microscopy (ExM) for Multiplexed Fluorescence
The principle behind expansion microscopy (ExM) is to physically pull apart the components of the cell to increase access. The sample is fixed and embedded into a swellable hydrogel matrix. The molecular components of the sample are then covalently bonded to the matrix itself. Structural proteins (such as cytoskeletal proteins) are cleaved so that the sample can swell equally in all dimensions, thus preserving the overall structure of the sample without deformation. The degree of expansion can be controlled by the osmolarity of the buffer. (Figure 2) Magnify Biosciences has standardized the process of linking common biomolecules (including proteins, membrane components and DNA) to the hydrogel in a commercially available kit called the Magnify Expansion Kit. They have expanded the chemistry to include linkages to bacterial and fungal components particular to these organisms in their next product under development called mMagnify. According to Aleks Klimas, Co-founder and CSO of Magnify Biosciences, “Magnify can effectively relieve steric hindrance in antibody binding to different epitopes within protein complexes. The molecular decrowding achieved through expansion physically separates biomolecules within the gel, improving antibody accessibility to previously masked epitopes.”
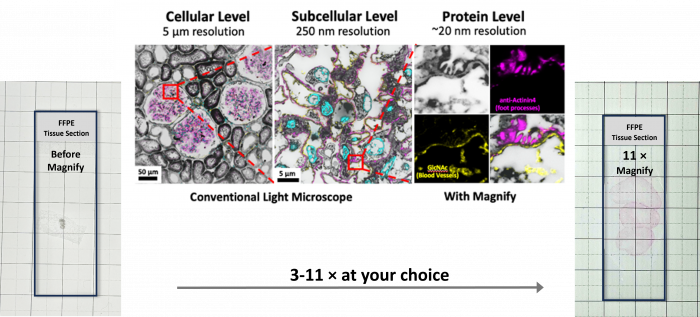
Figure 2: Mouse colon formalin-fixed paraffin-embedded (FFPE) slide before (left) and after Magnify expansion (middle and right). For subcellular imaging, users can choose an expansion factor between 3× and 6× by adjusting the salt concentration in the imaging buffer (middle). Full expansion up to 11× is achieved by placing the gel in pure water (right). The expanded gel remains colorless and transparent; a protein stain was applied to highlight the expanded mouse colon tissue (courtesy of Magnify Biosciences)
Another advantage of tissue expansion is improved diffusion of labels into thick samples. Especially for large molecules like antibodies, as the matrix swells, physical obstructions are reduced, increasing the diffusion rate. (Park, 2023) Optically, ExM enables super-resolution-like image acquisition with traditional, diffraction-limited fluorescent microscopes present in nearly every structural biology lab. (Figure 3) As the tissues expand, the refractive index of the tissue begins to approach that of the buffer, which also reduces aberrations in z-sectioning for confocal, multiphoton and other fluorescence microscopy techniques.
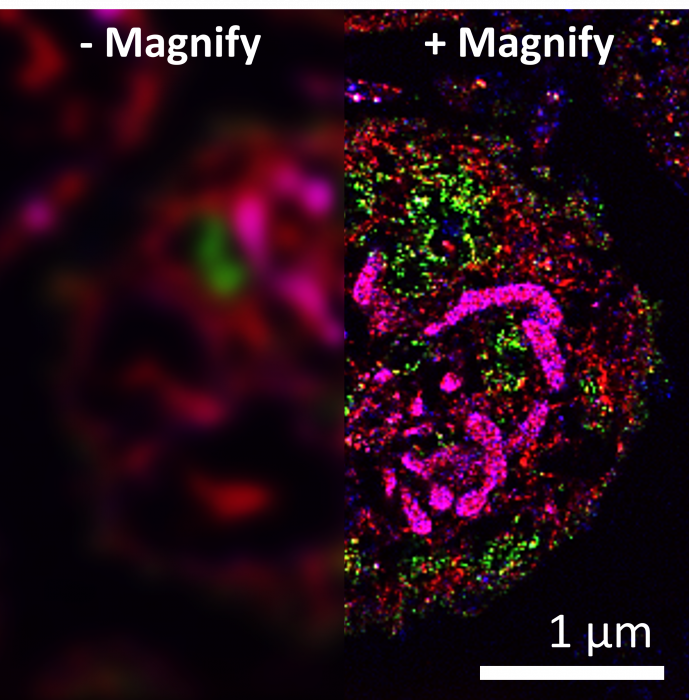
Figure 3. Human platelet cell before (left) and after (right) a 10x expansion using the Magnify Expansion Kit. Mitochondria were stained with the lipid stain DiD, revealing cristae structures. A green fluorescent lectin stain and a red pan-protein stain were used to highlight various subcellular structures. (courtesy of Magnify Biosciences)
Aleks Klimas continues, “Magnify stands out as a universal, high-expansion, and robust expansion microscopy method that works across diverse sample types and fixation methods while preserving biomolecules and enabling post-expansion staining. This makes it a powerful alternative to existing protocols, especially for applications requiring high-plex imaging in challenging samples like FFPE tissues and infected tissues. Our kits provide validated and tested reagents, ensuring reliable sample expansion without the need for users to source materials independently.”
Cyclical Multiplexed Immunofluorescence and Sterics
In cyclical multiplexed immunofluorescence, groups of antigens are labelled, imaged, stripped or bleached, followed by additional rounds of labelling for other sets of antigens. Learn more about cyclical immunofluorescence. When a chemical or photobleaching method is used between labelling steps to remove fluorescent signal, sterics can play a role in subsequent staining steps because the first round of antibodies remain bound to their antigens, taking up space and hindering access for later labelling steps. Physical stripping of bound antibodies between steps, removes the sterics issue, but it can cause tissue damage after repeated stripping cycles. The advantage of cyclical methods is that it can lead to very high-plex imaging – over 80-plex has been demonstrated.
Both ExM and VHH antibodies can be employed in cyclical multiplexed immunofluorescence protocols. Expansion enables extreme resolution with rapid antibody exchange through the expanded matrix, while effectively eliminating the space constraints of bleaching described above. (Cheng, 2023) VHH antibodies, given their small size and slightly lower affinity (ideal when using antibody stripping methods) makes them well-suited for cyclical immunofluorescence as well.
Supporting Your Research
FluoroFinder has developed a suite of tools to help you design and execute your experiments with ease. Use our Antibody Search function to find antibodies that have been validated for your chosen application and our Spectra Viewer to compare the spectral properties of more than 1,000 dyes alongside instrument-specific laser and filter configurations.
References
- Cai, R. K.-I. (2023). Whole-mouse clearing and imaging at the cellular lever with vDISCO. Nature Protocols, 18, 1197-1242. doi://doi.org/10.1038/s41596-022-00788-2
- Cheng, Z. S.-H. (2023). MicroMagnify: a multiplexed expansion microscopy method for pathogens and infected tissues. Advanced Science, 10(30), 2302249. doi:https://doi.org/10.1002/advs.202302249
- Matos, D. (2020, October 14). Steric hindrance: a practical (and frequently forgotten) problem in flow cytometry. Cytometry Part B: Clinical Cytometry, 100(4), 397. doi:https://doi.org/10.1002/cyto.b.21959
- Park, H. C. (2023). Size-dependent suppression of molecular diffusivity in expandable hydrogels: a single-molecule study. J. Phys. Chem. B., 127(14), 3333-3339. doi:https://doi.org/10.1021/acs.jpcb.3c00761
- Pothin, E. L. (2020). Brain delivery of single-domain antibodies: a focus on VHH and VNAR. Pharmaceutics, 12(10), 937. doi:https://doi.org/10.3390/pharmaceutics12100937
- Sayed, J. A. (2019). Multiplex immunohistochemistry: The importance of staining order when producing a validated protocol. Immunotherapy (Los Angel), 5(2). doi: 10.35248/2471-9552.19.5.157
- Zhang, Q. Z. (2023, November 29). Small antibodies with big applications: nanobody-based cancer diagnostics and therapeutics. Cancers (Basel), 15(23), 5639. doi:https://doi.org/10.3390/cancers15235639