The use of spectral flow cytometry is increasing due to the many benefits it affords.
Spectral flow cytometry is a relatively new technology that was developed to push the boundaries of traditional flow cytometry. First described in 2004 by researchers at Purdue University Cytometry Laboratories, spectral flow cytometry uses more sophisticated optics and detectors than those of conventional instruments to greatly increase the number of parameters that can be measured per cell. Specifically, rather than measuring a discrete range of wavelengths for each fluorophore, spectral flow cytometry records the full emission spectrum to offer improved flexibility for panel design. We comment on the advantages of spectral flow cytometry here, before highlighting three leading spectral flow cytometers being used for scientific research.
What is spectral flow cytometry?
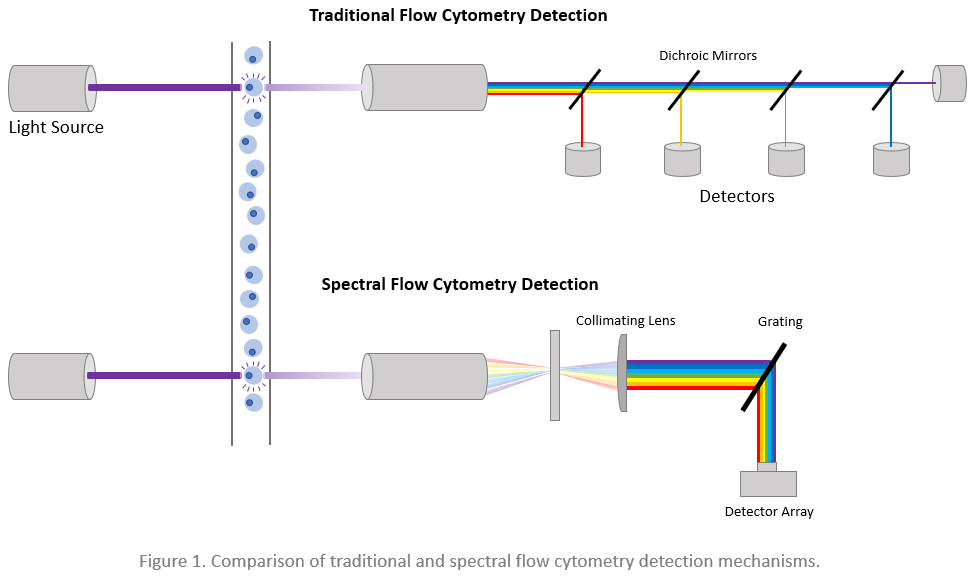
During both traditional and spectral flow cytometry, cells are stained with fluorophore-labeled antibodies before being directed by a fluidics system past an interrogation point where one or more lasers are focused. As each cell crosses the laser lines, it scatters the light and emits fluorescence from any cell-bound fluorophores, which produces signals that can be used for cellular identification. In a traditional flow cytometer, the light is guided by a series of dichroic mirrors and optical filters toward various individual detectors, each dedicated to capturing a discrete range of wavelengths. Spectral flow cytometers instead use gratings or prisms to separate the light, which is then measured across a detector array. This means that while traditional flow cytometers only detect one small snippet of the emission spectrum of each fluorophore, spectral cytometers record data for the emission spectrum of each fluorophore. By recording the entire emission spectrum of each fluorophore, spectral flow cytometry overcomes many limitations of conventional methods.
What are the advantages of spectral flow cytometry?
A major advantage of spectral flow cytometry is that it allows researchers to build larger panels by permitting fluorophores with similar emission spectra (e.g., GFP and YFP) to be combined in the same experiment. Using data from the entire emission spectrum of each fluorophore, the instrument’s internal software can identify distinct fluorophores with similar emission spectra via a process known as spectral unmixing. Once each unique fluorophore is identified, the instrument then determines how much of each fluorophore is bound per cell. An associated benefit of collecting more information per fluorophore is that experimental resolution and sensitivity are improved to ensure populations remain tight. Spectral flow cytometry is also able to treat autofluorescence as a separate parameter, thereby allowing for its subtraction from the total signal. This can be especially useful in detecting low abundance antigens on highly autofluorescent cells.
What instrumentation is currently available?
Since spectral flow cytometry was first described, several commercial instruments have reached the market. These include Sony Biotechnology’s SP6800, Cytek® Biosciences’ Aurora, and BD Biosciences’ FACSymphony™, all of which are cited in a growing number of publications.
Sony SP6800
Sony Biotechnology’s SP6800 Spectral Analyzer can be fitted with up to 3 lasers (405/488/638 nm) and detects emitted light across a 32 channel photomultiplier tube (PMT). It has been proven capable of measuring 16 fluorescent parameters with wavelengths ranging from 420nm to 800nm, and has recently been complemented by a newer model (the ID7000™) that can measure as many as 44 colors with wavelengths of 360 to 808 nm. Sony notes that key features of the SP6800 include enhancement of dim signal detection for better visualization of rare populations; a global standardization mode; and user-friendly software features such as automated alignment and laser delay, easy acquisition, and flexible analysis.
Cytek® Aurora
Cytek® Biosciences’ Aurora has up to 5 lasers (355/405/488/561/640 nm) and 64 fluorescence channels, enabling detection of as many as 40 emissions in the 365-829 nm range. The Aurora’s state-of-the-art optics and low-noise electronics are designed to enhance sensitivity and resolution, and their proprietary Coarse Wavelength Division Multiplexing (CWDM) semiconductor detector arrays improve the efficiency of spectrum capture. Cytek® Biosciences highlights the Aurora’s SpectroFlo® software, which provides an intuitive workflow spanning QC to data analysis and includes various tools to simplify running applications.
BD FACSymphony™
BD Biosciences’ FACSymphony™ Flow Cytometer allows researchers to select up to 10 lasers (from a pool of 25 possible options spanning 355 to 980 nm) and uses decagon arrays of PMTs for detecting as many as 50 different characteristics of a single cell. Most of the available lasers have multiple power ratings that can be adjusted and stored, and in many cases feature settings that are optimized for specific fluorophores. BD Biosciences promotes its expanding portfolio of Horizon Brilliant™ dyes for use with the FACSymphony® flow cytometer, as well as offers exclusive access to prototype dyes following instrument purchase.
Supporting spectral flow cytometry
Although panel design for spectral flow cytometry can seem daunting, FluoroFinder can help! Use our Panel Builder to build flow cytometry panels on your spectral instrument today. FluoroFinder’s Panel Builder is compatible with most spectral flow cytometers, and we are working hard to stay on top of all the emerging technologies. Additionally, use FluoroFinder’s Spectra Viewer in the new Spectral Flow Cytometry Mode to visualize various fluorophores across the full spectrum in the context of your instrument configurations.
Sign up for our eNewsletter to receive regular updates about spectral flow cytometry and other fluorescence-based techniques, including the latest instruments and fluorophores to become available.