Compensation is one of the most important but least understood aspects of multicolor flow cytometry
Multicolor flow cytometry allows researchers to characterize complex cellular populations by staining for several cell type specific markers at the same time. However, when more than a handful of colors is measured simultaneously, spillover can be a major issue, making compensation essential to avoid generating inaccurate results. This article provides a brief recap of basic fluorescence principles and explains why compensation is so important to the types of flow cytometry experiments being performed today.
Principles of fluorescence detection
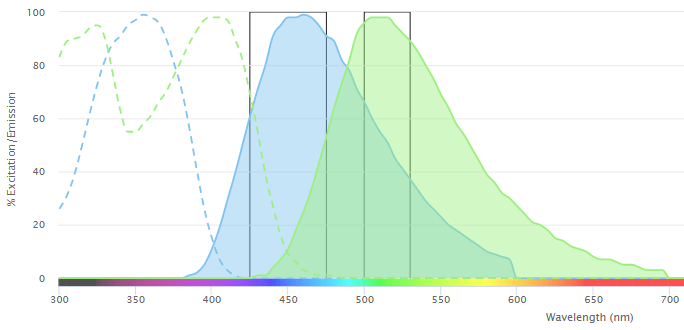
Fluorescence involves the transfer of energy from a photon of light to a fluorophore. This excites the fluorophore’s electrons, which must in turn release a photon of light to return to their ground state. Because the released photon has less energy than the original photon, it has a longer wavelength and is therefore a different color, a property that is exploited for fluorescence detection. Specifically, where fluorophores are conjugated to antibodies, they enable measurement of target analytes such as cell surface or intracellular markers with techniques like fluorescence microscopy or flow cytometry.
Fluorescence detection for flow cytometry
Flow cytometry uses fluorophore-conjugated antibodies for characterizing individual cells in suspension. As the cells pass through an interrogation point, lasers excite any cell-bound fluorophores, and the emitted light is measured by specialized detectors known as photomultiplier tubes (PMTs). Each time a photon of light hits a PMT, it generates a voltage pulse known as an event, which is used to produce a graphical representation of the sample. However, because a PMT will yield an event in response to any photon, the flow cytometer incorporates a series of dichroic mirrors and optical filters to restrict the light going toward each detector.
Spillover and compensation
Every fluorophore has distinct excitation and emission spectra, corresponding to the range of wavelengths at which it absorbs and emits light, respectively. Where a flow cytometry experiment is designed to measure just two or three fluorescent readouts, it is common practice to use fluorophores that are spectrally distinct. Yet, as panel size increases, overlap between the emission spectra of different fluorophores is inevitable. This can lead to one fluorophore being detected by the PMT of another – a phenomenon known as spillover that must be addressed to avoid misleading results.
Fortunately, irrespective of which fluorophores are used and how much of each fluorophore is present, the amount of spillover signal measured in a secondary detector is always proportional to the signal measured in the primary channel. This factor, known as the spillover coefficient, can therefore be calculated using single color controls, whereby running each fluorophore separately and measuring its fluorescent signal across all detectors enables compensation – the process of correcting for spillover to ensure only true staining is revealed.
Although compensation can be performed manually, automatic compensation is viewed as the gold standard approach since it addresses the issue of user bias. Yet, whichever strategy is employed, proper compensation involves following three simple rules. First, each fluorophore used for compensation must be matched to the corresponding fluorophore in the experimental setup. This is especially important when working with tandem dyes, which often exhibit significant lot-to-lot variability in terms of the photon transfer efficiency and degrade more readily than other fluorophores, increasing spillover into the emission channel of the donor.
Second, compensation controls must comprise both a negative (unstained) and a positive population for each color, with the positive population being as bright, or brighter, than any samples. Lastly, both positive and negative control populations should exhibit the same level of background fluorescence for each parameter being investigated such that an additional source of variability is not introduced. For example, if CD4+ T cells are used for the positive population, CD4– T cells (not CD4– B cells) should be used for the negative population. It is also recommended that compensation is performed for every experiment; compensation settings should not be applied from one experiment to another.
The critical importance of panel design
With flow cytometry increasingly being used for complex, multiparameter analyses, panel design is critical to minimize spillover and the need for compensation. A well-constructed multicolor flow cytometry panel will spread fluorophores out across as many lasers and detectors as possible, which is where FluoroFinder’s Panel Builder can help. By collating the fluorophore and antibody offerings of >60 suppliers in a single resource, our Panel Builder saves time spent trawling individual antibody manufacturer’s websites for information and can be used in conjunction with our Spectra Viewer to quickly compare over 1,000 fluorophores from all suppliers in one intuitive platform.
Sign up for our eNewsletter to receive regular updates about fluorescence-based techniques, including the very latest products for flow cytometry.