Understanding Flow Cytometry Beads
Immunobeads, or antibody-coated nanoparticles, are useful for a variety of flow cytometry-related applications. Here we outline the most commonly used bead types and explain how they can be used for cytometer calibration, compensation determination, and even cell sorting.
- Compensation “Capture” Beads
- Magnetic Beads”
- Quality Control Beads/Calibration Particles
- Counting Beads
- PMT Voltage Optimization Beads
1. Compensation “Capture” Beads When excited fluorochromes emit photons across a range of wavelengths, some may spillover to a second detector causing samples to appear double positive. Although FluoroFinder’s SpectraViewer helps you to minimize your spectral overlap during panel design, compensation is often required. Researchers perform fluorescence compensation to adjust for this spectral overlap and ensure that the fluorescence being measured comes from the correct fluorochrome. Compensation beads are homogeneous microparticles with low-level autofluorescence that bind multiple isotypes/species to produce a strong positive from which to calculate your compensation matrix. Positive control beads bind the antibodies in your panel, whereas negative control beads do not bind any antibody. Compensation beads are ideal for use in multicolor flow cytometry experiments on limited samples or when staining proteins with low expression levels. They are also particularly useful when working with tandem dyes, as lot-to-lot variability and storage conditions can affect their spectral properties Proper compensation requires the fluorochromes in the compensation control to exactly match those used in the experiment. Furthermore, the controls must be brighter than the experimental samples. The best practice is to use bright capture beads for compensation controls with matching colors to your experiment panel. When performing a compensation control, it may be necessary to reduce the amount of fluorochrome on the beads. Start by using 1/10 the amount of antibody on the beads as on the cells. Remember that your compensation signal must be at least as bright as the signal from your cells. However, if your compensation control runs off-scale, then simply re-stain the beads with less antigen. Some of the most commonly used capture beads include:
2. Magnetic Beads  Commonly used in immunoprecipitation applications, superparamagnetic nanoparticle beads are also useful for immunomagnetic cell sorting. These beads are particularly useful as a preliminary sort prior to flow cytometry analysis, or when running cells with strong intrinsic fluorescence which may interfere with fluorescent activated cell sorting techniques. Some of the most commonly used magnetic beads include:
Commonly used in immunoprecipitation applications, superparamagnetic nanoparticle beads are also useful for immunomagnetic cell sorting. These beads are particularly useful as a preliminary sort prior to flow cytometry analysis, or when running cells with strong intrinsic fluorescence which may interfere with fluorescent activated cell sorting techniques. Some of the most commonly used magnetic beads include:
3. Quality Control Beads /Calibration Particles  During cytometer installation and maintenance services, your machine is calibrated and optically aligned by a technician using quality control beads with varying sizes/fluorescence levels. Core staff is then responsible for tracking the instrument’s performance over time. Cytometers usually include an automated QC testing system that is recommended to be run daily using commercially available calibration particles. Typical QC testing involves running alignment, sensitivity, and fluidic quality control beads at known laser wattage/detector voltage levels to ensure that the median fluorescence intensity (MFI) measurement is consistent. QC testers should note any increase in the amount of voltage required to get the desired MFI reading. A required voltage increase across all detectors for a given laser often indicates a loss of laser power. However, if the reading is isolated to a single PMT, then a failing detector may be suspected. Additionally, QC testers should measure the coefficient of variation (CV) to determine the amount of variability relative to the mean of bead populations. Increased CV may indicate sensitivity loss possibly due to laser misalignment problems with the detection optics. Some of the most commonly used QC beads include:
During cytometer installation and maintenance services, your machine is calibrated and optically aligned by a technician using quality control beads with varying sizes/fluorescence levels. Core staff is then responsible for tracking the instrument’s performance over time. Cytometers usually include an automated QC testing system that is recommended to be run daily using commercially available calibration particles. Typical QC testing involves running alignment, sensitivity, and fluidic quality control beads at known laser wattage/detector voltage levels to ensure that the median fluorescence intensity (MFI) measurement is consistent. QC testers should note any increase in the amount of voltage required to get the desired MFI reading. A required voltage increase across all detectors for a given laser often indicates a loss of laser power. However, if the reading is isolated to a single PMT, then a failing detector may be suspected. Additionally, QC testers should measure the coefficient of variation (CV) to determine the amount of variability relative to the mean of bead populations. Increased CV may indicate sensitivity loss possibly due to laser misalignment problems with the detection optics. Some of the most commonly used QC beads include:
4. Counting Beads  Counting beads come in multiple sizes and may not even be fluorescent, but can be used to calculate the sample cell concentration. Researchers should set the stop gate to acquire a set number of events and then run the counting beads’ predetermined concentration. This measurement will allow researchers to accurately calculate the sample concentration. Some of the most commonly used counting beads include:
Counting beads come in multiple sizes and may not even be fluorescent, but can be used to calculate the sample cell concentration. Researchers should set the stop gate to acquire a set number of events and then run the counting beads’ predetermined concentration. This measurement will allow researchers to accurately calculate the sample concentration. Some of the most commonly used counting beads include:
6. PMT Voltage Optimization Beads  As their name suggests, PMT voltage optimization beads are used to optimize the photomultiplier tubes (PMT) voltage settings of a flow cytometer. These detector tubes convert emitted photons to an electronic signal that can then be read as data. Researchers must therefore determine the minimum voltage required for each detector to ensure sufficient signal boost beyond any underlying electronic noise. PMT voltage optimization beads can be used during routine flow cytometer calibration to measure both sensitivity and linearity. Sensitivity reading will help detect any dye or light contamination, whereas the linearity reading will determine if the logarithmic amps and PMTs are functioning properly. This test involves running a voltage series on each PMT and then plotting the coefficient of variation against the voltage range.
As their name suggests, PMT voltage optimization beads are used to optimize the photomultiplier tubes (PMT) voltage settings of a flow cytometer. These detector tubes convert emitted photons to an electronic signal that can then be read as data. Researchers must therefore determine the minimum voltage required for each detector to ensure sufficient signal boost beyond any underlying electronic noise. PMT voltage optimization beads can be used during routine flow cytometer calibration to measure both sensitivity and linearity. Sensitivity reading will help detect any dye or light contamination, whereas the linearity reading will determine if the logarithmic amps and PMTs are functioning properly. This test involves running a voltage series on each PMT and then plotting the coefficient of variation against the voltage range. 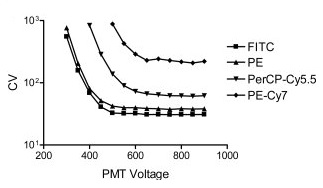 “The resulting curves have a predictable shape (see figure above), with CV initially decreasing as gain is increased, until sufficient voltage is applied such that the CV is stabilized with minimal electronic noise contribution. The inflection point of each curve can be taken as a reasonable minimum voltage to use for optimal resolution sensitivity when the brightness of the unstained or dim cells is similar to that of the standard particles” (Maecker, et al. 2006). Some of the most commonly used PMT voltage beads include Miltenyi Biotec Spherotech References: Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006 Sep 1;69(9):1037-42.
“The resulting curves have a predictable shape (see figure above), with CV initially decreasing as gain is increased, until sufficient voltage is applied such that the CV is stabilized with minimal electronic noise contribution. The inflection point of each curve can be taken as a reasonable minimum voltage to use for optimal resolution sensitivity when the brightness of the unstained or dim cells is similar to that of the standard particles” (Maecker, et al. 2006). Some of the most commonly used PMT voltage beads include Miltenyi Biotec Spherotech References: Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006 Sep 1;69(9):1037-42.





