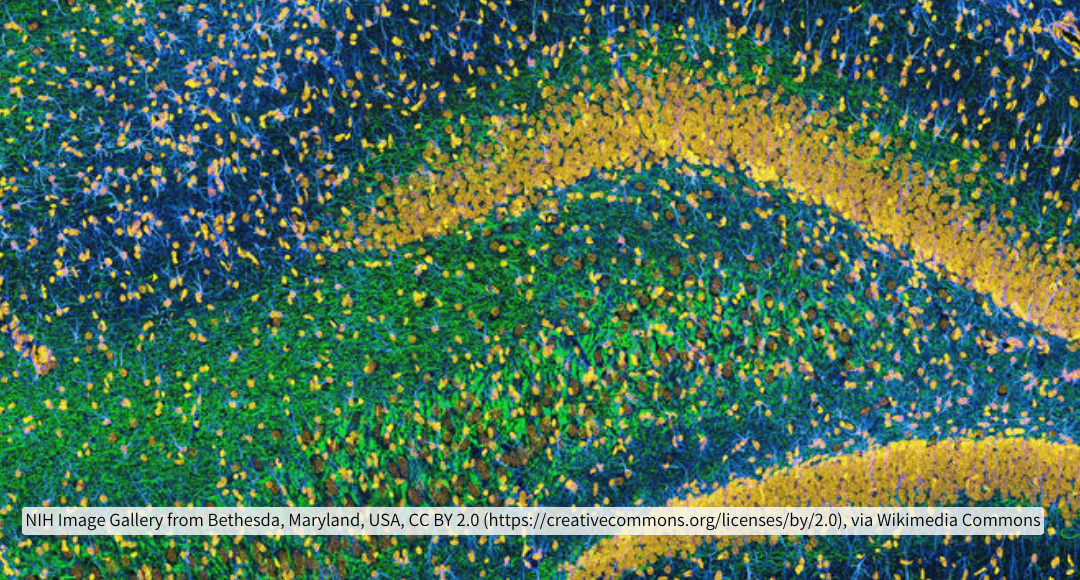
The cerebral cortex is between 2-4 millimeters thick depending on the region of the brain. In one cubic millimeter of human temporal cortex, there are 57,000 cells, 150 million synapses and 230 millimeters of blood vessels1. The typical thickness of a tissue slice for immunohistochemistry for widefield microscopy is 3-10 micrometers and 40 micrometers for confocal microscopy. That is a 1000 times reduction in the potential depth of analysis. All of those connections, reduced. In the brain, what is most important if it isn’t to understand how it is connected?
Some fundamental issues make imaging deep into a tissue problematic. Rather than write a book on the subject, give this review in the journal Cell2 a read and I’ll give simply a short synopsis of how multiphoton microscopy helps overcome two basic barriers to whole or deep tissue imaging. The first issue is associated with overcoming limited penetration of light and reagents into tissue and second is overcoming high background associated with endogenous autofluorescence. Also, rather than rehash the technological advancements of widefield and confocal microscopy, let’s just say that confocal was a very important advancement in imaging for the ability to focus a laser at specific points along different planes within a tissue section (confocality) and collect only the light emitting from that single point, minimizing the impact of scattered light on the spatial resolution limit between two fluorescent features or points of light. Additionally, there is important continued work to resolve spherical aberrations in the z-axis planes of thicker slices of tissue so that 3D reconstructions are more accurate to reality.
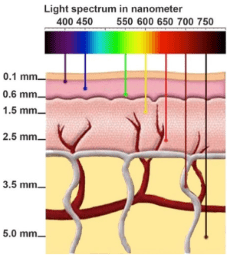 Take a look at this paper from Wang et al3 on the use of nanocrystals and C-dots in fluorescence microscopy. Even under optimized conditions, argon lasers (488/515nm) can only penetrate to ~0.6mm and the longer the wavelength of the excitation source, like the He-Ne laser at 633nm, the deeper the penetration. However, the light doesn’t need to just get into the tissue, as demonstrated in this graphic, but it needs to be focused on a single point (colimited) and the emission of the fluorescent probe needs to escape the tissue to be detected as well. An example from a 2011 paper by Clendenon et al determined that at 100 mm deep, the intensity of fluorescent emission was 22% of the maximum mean fluorescence intensity (MFI) for traditional confocal and 27% for multiphoton4. Multiphoton microscopy employs excitation energy wavelengths between 800-1000nm, very long wavelengths and thus very low energy. As a single photon, this is not sufficient to excite traditional fluorescent probes. However, double that. Make the photons able to simultaneously interrogate a fluorescent molecule at the same point in space (colimited) and time (coherent) using a pulsed laser, and the amount of energy the fluorophore experiences doubles. In an over-simplified way, two photons that coincidently hit a fluorophore, each at 800nm, and it will feel like 400nm to the fluorophore and provide enough energy to transition it to an excited state with subsequent fluorescence emission. Quite a feat of engineering to coordinate that concert of photons.
Take a look at this paper from Wang et al3 on the use of nanocrystals and C-dots in fluorescence microscopy. Even under optimized conditions, argon lasers (488/515nm) can only penetrate to ~0.6mm and the longer the wavelength of the excitation source, like the He-Ne laser at 633nm, the deeper the penetration. However, the light doesn’t need to just get into the tissue, as demonstrated in this graphic, but it needs to be focused on a single point (colimited) and the emission of the fluorescent probe needs to escape the tissue to be detected as well. An example from a 2011 paper by Clendenon et al determined that at 100 mm deep, the intensity of fluorescent emission was 22% of the maximum mean fluorescence intensity (MFI) for traditional confocal and 27% for multiphoton4. Multiphoton microscopy employs excitation energy wavelengths between 800-1000nm, very long wavelengths and thus very low energy. As a single photon, this is not sufficient to excite traditional fluorescent probes. However, double that. Make the photons able to simultaneously interrogate a fluorescent molecule at the same point in space (colimited) and time (coherent) using a pulsed laser, and the amount of energy the fluorophore experiences doubles. In an over-simplified way, two photons that coincidently hit a fluorophore, each at 800nm, and it will feel like 400nm to the fluorophore and provide enough energy to transition it to an excited state with subsequent fluorescence emission. Quite a feat of engineering to coordinate that concert of photons.
However, as mentioned in the Clendenon paper, there is only a 5% recovery of intensity using multiphoton alone and there are many strategies to increase efficiency besides these that I mention. Deep tissue imaging is also limited by the abundance of endogenous autofluorescence like vitamins, metabolic cofactors, melanin, cellular adhesion molecules, lipids and lipofuscin. If the tissue or organ can be removed or sliced, one could apply clearing agents like CLARITY, DISCO or CUBIC to delipidate the sample, creating a more transparent tissue with a matching refractive index more suitable for imaging. An excellent review of these clearing methods can be found in this Nature Reviews Neuroscience paper by Ueda et al5.
However, even if tissue cannot be cleared first, multiphoton offers advantages in dealing with the high background of autofluorescence in that there are not many natural sources of fluorescence that are excited by such long single wavelengths as are employed in multiphoton. The excitation peak of most natural fluorescent molecules is between 250-400nm which translates to 700-800nm 2-photon (2P) excitation in multiphoton. Therefore, as the single wavelength of 800nm navigates through the tissue heading for its coincident collision with other photons at the focal point, the autofluorescent molecules are not absorbing the light as it infiltrates through the tissue. The intensity of excitation isn’t being diminished as dramatically by absorption of the excitation energy and the energy isn’t sufficient to cause unwanted signal from autofluorescent molecules.
In addition to these advantages, multiphoton can be coupled to lifetime imaging. Rather than the emitted wavelength being detected as a relative intensity unit, the detection modality could instead measure the decay rate or the average time a fluorophore spends in its excited state. Lifetime provides a third dimension to further resolve the identity of a source of light as it can be a fingerprint unique to the source of fluorescence.
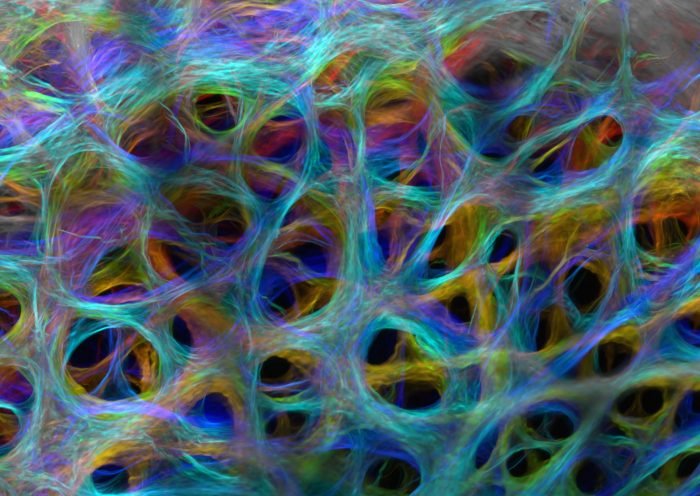
Image of Distinction, Nikon Small World Competition. Optic Nerve Head Collagen of a Pig by Susannah Waxman and Dr. Ian Sigal at Univ Pittsburgh.
Every year I wait for the results of the Nikon Small World imaging contest to be announced. This image is a 2024 contender that employed multiphoton to image the collagen network of the optic nerve head, the physiological support for the eye. Beautiful. Also, the best education one can receive about how multiphoton works is directly from the vendor. I recommend following the links below for a wealth of educational material.
Supporting Your Research
Here at FluoroFinder, we are developing tools to aid in microscopy assay design. For example, our Dye Directory contains information about the extinction coefficient, molecular weight and quantum efficiency of different fluorophores and fluorescent proteins, in addition to antibody products, conjugation kits and services available from different vendors. An example of that is the Alexa Fluor 488 snapshot below. We are also working on a panel building tool to help organize reagents for Cyclic IF applications. 2025 is sure to bring with it new tools from FluoroFinder to help with all of your microscopy application needs.

Links to Recommended Educational Material
- https://www.olympus-lifescience.com/en/microscope-resource/primer/techniques/fluorescence/multiphoton/multiphotonintro/
- https://www.microscopyu.com/techniques/multi-photon/multiphoton-microscopy
- https://www.leica-microsystems.com/science-lab/life-science/principles-of-multiphoton-microscopy-for-deep-tissue-imaging/
References
- Shapson-Coe A, Januszewski M, Berger DR, et al. A petavoxel fragment of human cerebral cortex reconstructed at nanoscale resolution. Science. 2024;384(6696):eadk4858. doi:10.1126/science.adk4858
- Xu C, Nedergaard M, Fowell DJ, Friedl P, Ji N. Multiphoton fluorescence microscopy for in vivo imaging. Cell. 2024;187(17):4458-4487. doi:10.1016/j.cell.2024.07.036
- Wang J, Liu G, Leung KC, Loffroy R, Lu PX, Wang YX. Opportunities and Challenges of Fluorescent Carbon Dots in Translational Optical Imaging. Curr Pharm Des. 2015;21(37):5401-5416. doi:10.2174/1381612821666150917093232
- Clendenon SG, Young PA, Ferkowicz M, Phillips C, Dunn KW. Deep tissue fluorescent imaging in scattering specimens using confocal microscopy. Microsc Microanal. 2011 Aug;17(4):614-7. doi: 10.1017/S1431927611000535. Epub 2011 Jun 24. PMID: 21729357; PMCID: PMC4428593.
- Ueda HR, Ertürk A, Chung K, Gradinaru V, Chédotal A, Tomancak P, Keller PJ. Tissue clearing and its applications in neuroscience. Nat Rev Neurosci. 2020 Feb;21(2):61-79. doi: 10.1038/s41583-019-0250-1. Erratum in: Nat Rev Neurosci. 2020 May;21(5):298. doi: 10.1038/s41583-020-0291-5. PMID: 31896771; PMCID: PMC8121164.
- Dancik Y, Favre A, Loy CJ, Zvyagin AV, Roberts MS. Use of multiphoton tomography and fluorescence lifetime imaging to investigate skin pigmentation in vivo [published correction appears in J Biomed Opt. 2013 Feb;18(2):29802]. J Biomed Opt. 2013;18(2):26022. doi:10.1117/1.JBO.18.2.026022