Historically, fluorescence microscopy was limited by the number of parameters that could be imaged on a single sample due the spectral overlap of the fluorophores available at the time. There was a prevailing dogma that only one fluorophore could be assigned to each laser, or bandpass of excitation energy) as an attempt to sequentially image spectrally separated reagents one at a time. Commonly, the imaging application using a widefield microscope with mercury arc or halide lamps typically involve applying a nuclear counterstain like Hoeschst or DAPI, along with two additional channels: one green and one “red” (although the emitted light might range from 580nm to 630nm, which could actually appear yellow, orange, or red). The limited number of parameters that could be imaged on a single tissue preparation is problematic.
In the early stages, the introduction of two imaging components helped to add a fourth parameter to imaging experiments. First, the implementation of X-Cite lamps, LEDs, and lasers with an excitation peak between 630-645nm expanded possibilities. Second, the increasing use of more sensitive cameras and PMTs made it more efficient to detect emitted photons greater than 660nm. Although the dogma was still a 1:1 ratio of reagent and excitation line, the implementation of deep red/NIR illumination provided a valuable alternative to the challenge of imaging between 350-520nm, a spectral range complicated by high autofluorescence of samples. Additionally, the enhanced sensitivity of detectors enabled the use of directly conjugated, albeit dimmer, primary antibodies, since species-dependent secondary detection limited the number of primary antibodies able to be simultaneously exclusively stained.
In 2001, Zeiss released the LSM 510 Meta, a wildly popular confocal microscope that implemented spectral imaging alongside mathematical linear unmixing, also known as spectral unmixing, to compensate for the spectral spillover of overlapping fluorophore emission. Spectral unmixing works to extract the spectral fingerprint of both endogenous and exogenous fluorescence in situ using unstained and single-color controls. This innovation was revolutionary for tackling the persistent problem posed by autofluorescence in tissue. Essentially, each photon has a certain probability of belonging to a particular fluorophore based on the statistical distribution of photons across an array of spectrally distinct channels. The spectral fingerprints of fluorophores can change depending on the microenvironment of the fluorophore, and this technique requires a sufficient number of photons in each channel to accurately represent the spectral characteristics. Despite these risks, the LSM 510 Meta enabled the application of six or more parameters per sample with three or four laser excitation lines, exceeding previous limitations. Fast forwarding 23 years, reagent design, microscope engineering and image analysis have transformed the landscape of high-parameter microscopy. In this article, we will overview a few of the solutions enabling high-parameter imaging.
CODEX
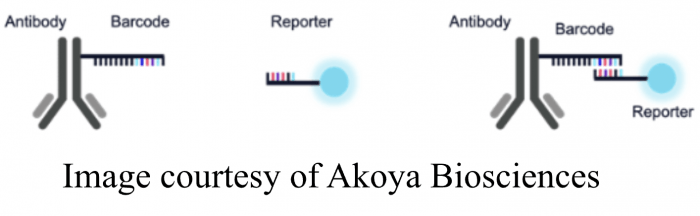
The first solution to the challenge of high-parameter imaging involved overcoming the dependence on species-dependent secondary detection or the reliability issues associated with direct conjugation kits used at the bench. In 2018, Garry Nolan (1) introduced the CODEX (CO-Detection by antibody IndEXing) system, which uses complementary oligo sequences to couple antibodies with fluorophores as an alternative to direct antibody conjugation. This technology is employed by instrument suppliers like Akoya, whose PhenoCycler, a slide-scanning imaging system for staining and imaging cyclic rounds of immunofluorescence, removes the detection moiety or reporter between each round. Akoya’s PhenoImager combines CODEX method with spectral detection.
IBEX and Cyclic Multiplexed-IF
Another significant challenge of cyclic multiplex IF (cmIF) was removing fluorescent signals between rounds of imaging. Photobleaching is a method of destroying the integrity of a fluorophore by oxidation from light in an aqueous environment. However, it alone is not sufficient for complete and uniform quenching of fluorescence in a reasonably short timeframe. Ron Germain’s lab has pioneered a method called IBEX (iterative bleaching extends multiplexity), which combines the reducing agent lithium borohydride with light to reduce the time required for bleaching (2), to address this challenge. This method is a supplier-independent, open-source method for any lab to apply cmIF.
Some commercial systems rely upon both photobleaching and non-photobleaching methods of label removal. Although a proprietary mechanism, Miltenyi offers antibodies and fluorophores engineered with a release mechanism called REA-lease (3). Upon application of a proprietary buffer, the fluorophore is “released” from the antibody and washed away. Miltenyi’s MACSima imaging cyclic staining technology stains and images on-slide, streamlining the staining and image processing.
Various stripping buffer combinations have been used to challenge the problem of antibody and fluorophore removal. Examples include oxidizing solutions like KMn04/H2SO4 (4), H2O2, or enzymatic methods using engineered cleavage sites for specific enzyme-mediated removal. Cleavable fluorescent tyramide (CFT) is another enzymatic method, which uses a chemical reaction between PTA and TCEP for stripping (5). Most of these methods use microwave conditions to accelerate the procedure. The literature contains excellent attempts to address this problem.
Furthermore, many platforms employ spectral imaging between cycles to assess for changes in autofluorescence resulting from treatment conditions, effectively recalibrating the background of each parameter. The implementation of these advanced techniques has led to platforms and applications capable of analyzing 30-50 proteomic markers. Coupled with artificial intelligence learning, this is a new era of high-multiplexed fluorescent imaging and digital pathology.
References
- Goltsev Y, Samusik N, Kennedy-Darling J, Bhate S, Hale M, Vazquez G, Black S, Nolan GP. Deep Profiling of Mouse Splenic Architecture with CODEX Multiplexed Imaging. Cell. 2018 Aug 9;174(4):968-981.e15. doi: 10.1016/j.cell.2018.07.010. Epub 2018 Aug 2. PMID: 30078711; PMCID: PMC6086938.
- Radtke AJ, Kandov E, Lowekamp B, Speranza E, Chu CJ, Gola A, Thakur N, Shih R, Yao L, Yaniv ZR, Beuschel RT, Kabat J, Croteau J, Davis J, Hernandez JM, Germain RN. IBEX: A versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues. Proc Natl Acad Sci U S A. 2020 Dec 29;117(52):33455-33465. doi: 10.1073/pnas.2018488117. Epub 2020 Dec 21. PMID: 33376221; PMCID: PMC7776876.
- Kinkhabwala A, Herbel C, Pankratz J, Yushchenko DA, Rüberg S, Praveen P, Reiß S, Rodriguez FC, Schäfer D, Kollet J, Dittmer V, Martinez-Osuna M, Minnerup L, Reinhard C, Dzionek A, Rockel TD, Borbe S, Büscher M, Krieg J, Nederlof M, Jungblut M, Eckardt D, Hardt O, Dose C, Schumann E, Peters RP, Miltenyi S, Schmitz J, Müller W, Bosio A. MACSima imaging cyclic staining (MICS) technology reveals combinatorial target pairs for CAR T cell treatment of solid tumors. Sci Rep. 2022 Feb 3;12(1):1911. doi: 10.1038/s41598-022-05841-4. PMID: 35115587; PMCID: PMC8813936.
- Glass G, Papin JA, Mandell JW. SIMPLE: a sequential immunoperoxidase labeling and erasing method. J Histochem Cytochem. 2009 Oct;57(10):899-905. doi: 10.1369/jhc.2009.953612. Epub 2009 Apr 13. Erratum in: J Histochem Cytochem. 2010 Oct;58(10):939. PMID: 19365090; PMCID: PMC2746723.
- Pham T, Nazaroff CD, Labaer J, Guo J. Ultrasensitive and Multiplexed Protein Imaging with Cleavable Fluorescent Tyramide and Antibody Stripping. Int J Mol Sci. 2021 Aug 11;22(16):8644. doi: 10.3390/ijms22168644. PMID: 34445351; PMCID: PMC8395465.