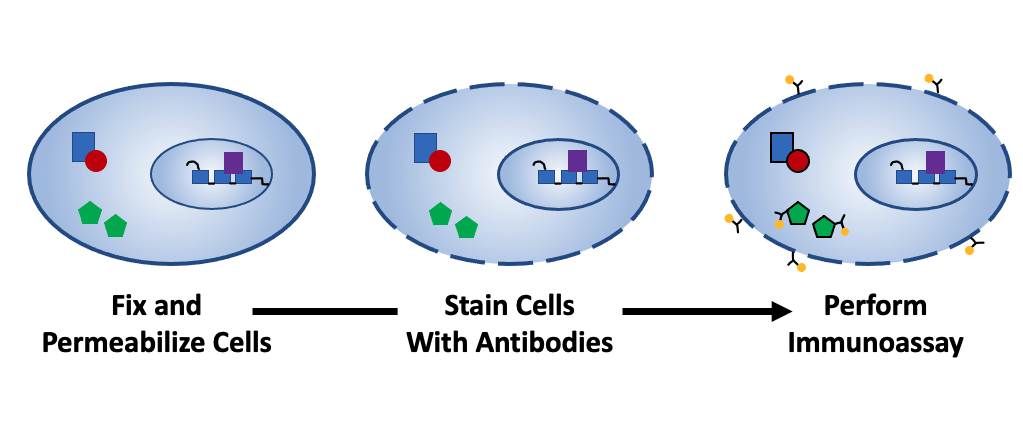
Fixation and permeabilization are key protocol steps for several core immunoassay techniques
Immunoassay techniques such as flow cytometry, immunocytochemistry (ICC), and immunohistochemistry (IHC) often require that samples be fixed and/or permeabilized prior to staining with antibody reagents. While fixation and permeabilization serve different purposes, both must be carefully optimized for the sample type and antibodies being used. Here, we discuss the science behind fixation and permeabilization and provide guidance for method selection.
What is fixation?
Fixation is the chemical preservation of sample material such as single-cell suspensions, plated cells, and tissue sections. It serves to block the activity of endogenous proteases, thereby preventing sample degradation, and provides a means of stabilizing the sample architecture and protecting against microbial contamination. Critically, fixation should ensure that the sample remains as close as possible to its native state for accurate conclusions to be drawn from experimental data.
What are some common fixatives?
Fixatives are broadly classified into two main groups. Aldehyde-based fixatives such as formaldehyde, glutaraldehyde, and formalin (an aqueous solution of formaldehyde containing a low concentration of methanol as a stabilizer) are most commonly used, and work by creating bonds between lysine residues to cross-link sample proteins. Aldehyde-based fixation hardens the sample to help protect against any adverse effects of downstream processing, as well as allows for long term storage of material such as cell pellets and excised tissue if these are subsequently embedded in paraffin.
On the flip side, aldehyde-based fixation can chemically alter target epitopes to prevent antibodies from binding, and often requires antigen retrieval for IHC, specifically if the fixative solution has been incubated with the sample for an extended period of time. A further limitation of aldehyde-based fixation is that it can generate unwanted autofluorescent by-products, creating problems for techniques using immunofluorescent detection. This issue can be addressed by selecting fluorophores with longer emission wavelengths for staining formaldehyde-fixed samples since most autofluorescence is emitted in the 350-550 nm range.
Alternatively, Alcohol-based organic solvents such as methanol, ethanol, and acetone work by dehydrating the sample material, which causes protein denaturation and precipitation. These types of fixatives eliminate the need for a separate permeabilization step when detecting intracellular antigens. However, alcohol-based fixation can lead to soluble proteins being washed away and can alter the tertiary structure of target epitopes due to the disruption of hydrophobic bonds.
What is permeabilization?
Permeabilization is the process of providing antibody reagents with access to intracellular antigens. It works by disrupting cell membranes and/or the membranes of organelles, meaning that the choice of permeabilizing agent will largely be driven by where the target antigen is located. Often, a low concentration of the permeabilizing agent will be included in blocking solutions, wash buffers, and antibody diluents. This is to ensure antibody access to intracellular targets is maintained throughout the course of the experiment and to reduce non-specific binding (e.g., by disrupting hydrophobic interactions).
What are some common permeabilizing agents?
Permeabilization is typically performed with detergents or alcohol-based organic solvents, of which the latter both fix and permeabilize cells at the same time. Widely used detergents include Triton™ X-100 and Tween-20, which non-selectively permeabilize all lipid bilayers including the nuclear membrane, and saponin and digitonin, which differentially permeabilize cells and intracellular compartments based on the membrane cholesterol content.
Factors to consider for fixation and/or permeabilization
One important factor to consider when fixing and/or permeabilizing samples prior to immunostaining is whether the antibodies you intend to use are compatible with your chosen method. Antibody datasheets should include detailed protocols describing how the antibodies have validated for different applications. It is advised that researchers use the manufacturer-recommended conditions as a starting point for optimizing antibody use in their own model system.
A common mistake is to assume that an antibody which is validated for IHC staining of formalin-fixed paraffin-embedded (FFPE) tissue will work comparably with frozen (methanol-fixed) sample material – this is by no means the case. Researchers may also erroneously assume that extracellular and intracellular targets can be detected simultaneously. If antibodies to extracellular targets have not been validated for use with fixed and/or permeabilized samples, researchers are advised to stain for these first, before fixing, permeabilizing, and detecting intracellular targets.
Fixation and permeabilization can also impact fluorophore performance. For example, fluorescent proteins such as phycoerythrin (PE), allophycocyanin (APC), and the peridinin-chlorophyll-protein complex (PerCP) will be denatured by alcohol-based organic solvents, while most fluorescent dyes are able to withstand these reagents. Again, check with the manufacturer as to how different fluorophores have been tested.
Other factors to bear in mind when planning your experiment concern the fixation and permeabilization process – temperature, reagent concentrations, choice of diluent, and incubation times can all impact results. Once you have established optimal conditions for your particular experimental setup, be consistent with how you handle your samples since this will help to avoid variable results.
Supporting your research
Whether you’re performing flow cytometry, IHC, or any other technique involving sample fixation and/or permeabilization, FluoroFinder is here to help! Use our Antibody Search function to find antibodies that complement your chosen fix/perm agents, and visit our Spectra Viewer to determine compatible fluorophores for your instruments and select antibodies for your immunoassay.