Flow cytometry controls must address multiple sources of variation
Flow cytometry requires more controls than other immunoassay techniques because it accounts for a greater number of potential sources of variation. In addition to experimental controls that validate staining specificity and ensure assay consistency, researchers must also include controls to resolve cellular populations and optimize instrument performance. Ultimately, controls are critical to safeguard the reproducibility and provide confidence in the accuracy of flow cytometry data. FluoroFinder’s Panel Builder allows users to factor in controls while designing flow cytometry experiments and helps streamline viability dye selection.
Biological Controls
Biological controls consist of positive and negative samples, cells that are known to either express or lack expression of the antigen of interest. Examples of positive controls include characterized cell lines, cells transfected to ensure positive staining, and cells treated to stimulate marker expression. Negative controls also include characterized cell lines, as well as cells in which marker expression has been knocked out via CRISPR or RNAi. Researchers can use open access resources such as PAXdb and The Human Protein Atlas, antibody datasheets, and published data to identify relevant biological controls.
Reagent Controls
Reagent controls serve to ensure that flow cytometry reagents – especially antibodies and fluorophores – are properly functioning and being used at appropriate concentrations. Among these reagent controls, antibody titration is essential to avoid overusing antibodies, which can lead to non-specific binding, or underusing antibodies, which can reduce assay sensitivity. Additionally, researchers should perform lot-to-lot comparisons every time a batch of reagent is changed.
Isoclonal controls are also important to confirm that target cells are not binding to fluorophore labels. This involves staining the sample with each antibody conjugate in the presence of excess unlabeled antibody, then measuring the resultant fluorescence. A lack of fluorescence confirms the absence of non-specific fluorophore binding.
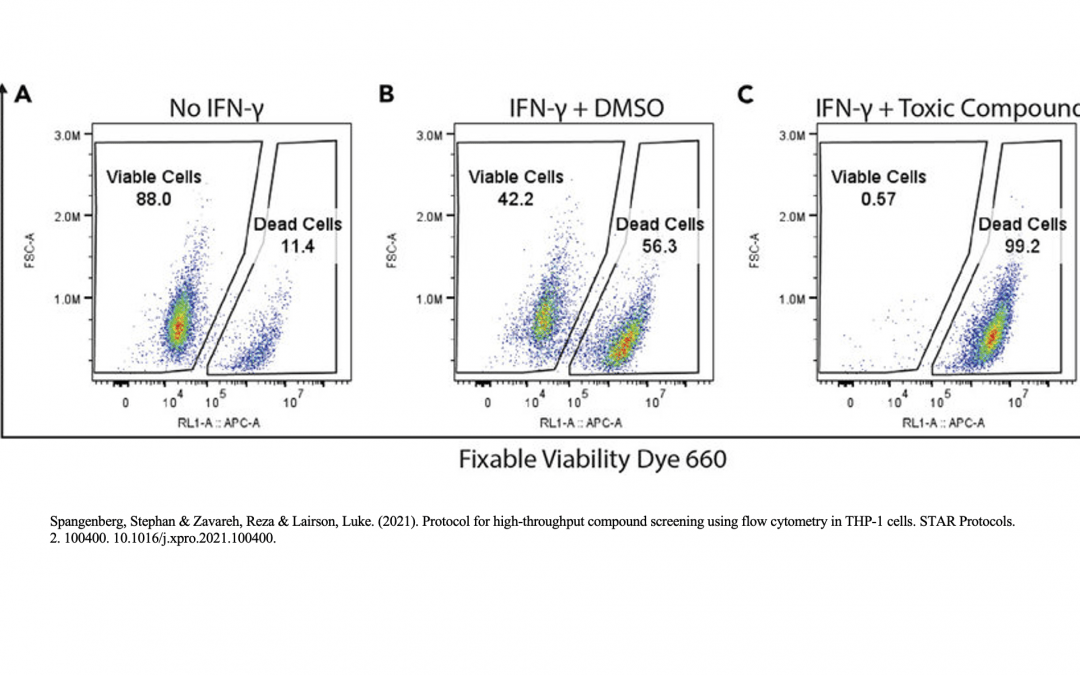
Viability Controls
The presence of dead cells and cellular debris in samples analyzed by flow cytometry gives rise to autofluorescence and non-specific antibody binding. This will make it difficult to detect weakly positive samples and rare populations during analysis. To minimize the presence of these unwanted cells, researchers can use viability controls to distinguish live cells from dead cells and debris. Common viability dyes include 4′,6-diamidino-2-phenylindole (DAPI), a blue nuclear stain which binds to dead cells with permeable membranes, and 7-amino actinomycin D (7-AAD), which fluoresces red upon DNA binding. Other examples include acridine orange/propidium iodide, which stains live cells green and dead cells red, and fluorescent conjugates of Annexin V, which bind the apoptosis marker phosphatidylserine and are often combined with a nuclear stain such as propidium iodide.
Unstained Controls
Unstained controls allow researchers to determine the level of cellular autofluorescence in samples and to set voltages and negative gates. They are produced by processing samples in the absence of antibody conjugates to ensure that differences between the stained and unstained samples are not due to how the cells have been handled.
Fluorescence Minus One (FMO) Controls
Fluorescence minus one controls (FMOs) are used to account for spectral overlap in multicolor flow cytometry panels. These controls involve staining samples with all but one of the fluorophores in the panel, then measuring the contribution of those fluorophores to the detection channel of interest. FMO controls are crucial for gating, particularly in detecting rare events or emerging antigens such as cell activation markers.
Single Color Compensation Controls
Compensation controls are used to determine a fluorophore’s spread into channels beyond that of its detection. These controls typically consist of either single fluorophore stained cells, preferred for larger samples with a highly-expressed target of interest, or antibody capture beads, preferred for targets with low expression. When preparing compensation controls, the fluorophores should exactly match those in the experiment (right down to the lot number). The measured fluorescence should be at least as bright as that of the sample. Also, the compensation algorithm should be performed with both a positive and negative population.
Isotype Controls
Isotype controls are antibodies that share the same heavy and light chains as the target antibody and are raised against a surface antigen not expressed by the cell type being studied. Although once the go-to negative control for flow cytometry experiments, isotype controls have recently fallen from favor for introducing an additional experimental variable.
Streamline Experimental Design and Execution
Designing a multicolor flow cytometry panel? FluoroFinder has developed a variety of resources to guide you through the panel design and reagent selection process. Our Panel Builder allows you to visualize the antibody offerings from all major suppliers in the context of your cytomter’s configuration and access our extensive selection of viability dyes. Our Antibody Search also helps you find antibodies validated for flow cytometry that are for your target antigens.
Sign-up for our eNewsletter to receive further updates about flow cytometry and other fluorescence-based techniques.