Fluorescent cellular analytical technologies allow us to “see” beyond what was historically possible with histological stains or morphological scatter profiles. In the early days, microscopy employed excitation sources like arc lamps, isolating specific wavelengths of light to excite single organic fluorophores as discreetly as possible. Flow cytometry, on the other hand, in order to increase the number of markers able to be detected and the speed of acquisition, instead began by employing 1-3 laser lines intended to excite multiple, specifically-designed tandem fluorophores with the proteins PE or APC acting as the donor fluorophore, that is the fluorophore receiving the excitation energy, and a donor fluorophore that in turn is excited by the energy of the donor fluorophore in a FRET (Förster Resonance Energy Transfer) relationship. However, fluorophores are not discreet and the panels we employ in flow cytometry are significantly more spectrally complex than when compensation was first introduced. Thus, there are two types of fluorescent spillover that plague flow cytometry. First is when the fluorophore has a wide emission range that spills over into a neighbor’s emission channel off the same laser, in this context called its unintended channel. Second is when the fluorophore is somewhat excited by other lasers besides its intended laser which is called cross-beam excitation. Compensation is a process to visually correct for either form of spillover, with the intention to increase the accuracy of analysis. In this article, we’ll discuss considerations for effective compensation in flow cytometry.
The Need for Compensation
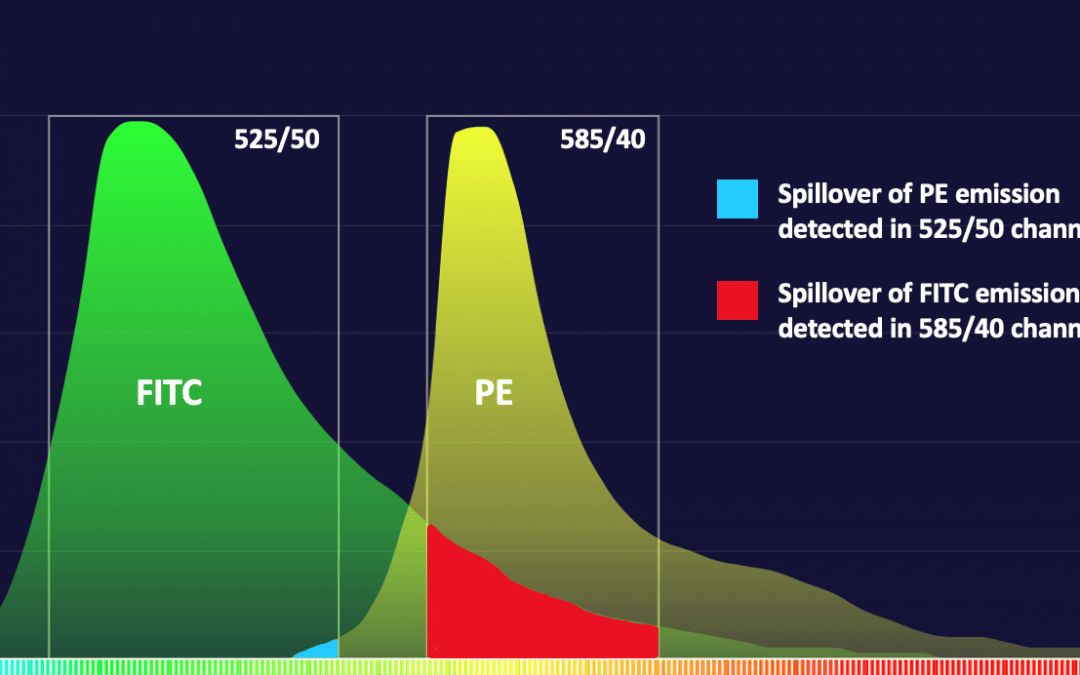
When a fluorophore’s emission spills into an unintended channel, the resulting bivariate analysis plot of the fluorophore spilling (the culprit) and the channel into which it spills (the victim) will display an unusual artifact of one or both populations arcing towards the other axis rather than in a straight path along its axis. Compensation is the mathematical correction factor that was derived from the spillover calculated using single color compensation controls and applied to both fluorophores to restore the correct alignment of the populations on all axes. Compensation does not change the data itself. The amplitude and percent positive statistics of a population must always remain the same. The visual correction of aligning a population on its axis prevents a distortion of the population that might impede accurate gating and the resolution of low abundant or double positive populations when doing bivariate gating.
Compensation Controls
To measure the amount of spectral spillover in each channel, single-stained cells or antibody-binding beads, each labeled with one of the fluorescent reagents used in the panel, are separately collected at the beginning of acquisition in order to apply compensation to the subsequent samples. It is also possible to acquire compensation controls, compute and employ compensation post-acquisition offline in analysis using software like FCS Express or FlowJo.
The accuracy of compensation is entirely dependent on the following good practices.
Fundamental Rules of Compensations
Use the same reagent as was used for the fully stained sample so the spectra match identically
This is relevant when using tandem dyes, which often have lot-to-lot variability and differential photobleaching that impact the efficiency of energy transfer between the donor and acceptor. This difference in efficiency changes the spectral distribution, specifically the ratio of emission into the primary channel of the donor and the primary channel of the acceptor. The precision of the ratio of intended and unintended emission is the data that will derive the compensation correction factor.
However, fluorophores like the Alexa Fluors, R718, FITC, BV421, BUV395, PE and APC etc are not tandems. Their emission profile will not change like tandems. If you run out of these reagents and don’t have enough to prepare a compensation control from the same reagent used in the assay, other antibodies conjugated with these non-tandem fluorophores will all have identical spectrum.
Also, avoid any enticement to use spectral files for compensation that are saved into a spectral library on the instrument. If these were not generated with the reagent used in the assay and on an instrument that is set-up and performing identically to when the files were generated, it will introduce inaccuracy. This method should only be used in case of emergency and full understanding that it may introduce error.
Whether the substrate of the compensation control is beads or cells, the autofluorescence of the unstained negative control must be identical to the rest of the compensation controls.
It is not uncommon to employ both antibody-capture compensation beads and cellular controls in the same experiment. However, in doing so, a negative control for both substrates are required since they have very different autofluorescent spectra. If you are accustomed to using a universal negative, that is a single negative control applied to all the compensation control calculations, the option needs to be clicked off if using both beads and cells. Also, if your cell sample is activated or diseased in any way, the autofluorescence of the unstained cell will change. It is good practice to acquire both a healthy and diseased/activated cell sample in the event it affects the accuracy of compensation and population gating.
The amplitude of the positive population of the compensation control must be the same or brighter than the signal you will see on the fully stained sample.
The correction factor (compensation value) is only as accurate as the amplitude of the staining used to calculate the standard curve of correction. If the cellular staining of a fluorescent reagent is very dim, it is better to use a compensation bead. For example, the amine-reactive fixable viability reagents are very popular. However, in freshly isolated human blood, viability can exceed 95% live. Thus, there are not many dead cells to get enough emission data to calculate an accurate correction factor. In this instance, there are a few possible solutions. You can heat kill the cells by putting the vial of PBMC on a 95°C hotplate for 5-10 min prior to labeling with the reagent. Or, you could also employ compensation beads that are specifically aminated to bind the live/dead fixable reagents. ArC beads from ThermoFisher and ViaComp beads from Slingshot are both good examples.
Compensation Controls cannot be contaminated with any other fluorescent signal or reagent.
This seems like a no-brainer, but contamination is frequent when using microtiter plates for staining, since the washing process can sometimes cause the wells to overfill or splatter into neighboring wells unintentionally. The contamination often isn’t detected until you are setting up compensation on the cytometer. Since most cytometers are in core facilities and not in your own lab, it can save you time and energy to bring a vial of fresh compensation beads and the reagents you used in the experiment with you (on ice and protected from light). If you see spillover from a comp control that does not represent the expected spectrum of that reagent, you can quickly re-stain a fresh compensation control with that reagent.
It’s all about accuracy. If the spectrum being used to calculate spectral spillover isn’t accurate for any of the above reasons, the compensation will not be accurate when applied to your important biological samples. You may hear an ill-advised recommendation to “fix” the compensation in analysis, but this is not good advice. First, we should ask WHY the compensation is not correct and see if there is a solution to fix the real underlying problem. Post-compensation changes to the compensation matrix will cascade artifacts to the spectral balance achieved in other channels. Fixing a problem in one spot may unintentionally lead to creating problems in others. In science, our results are only as reliable as our controls were accurate.
There is one saving grace. Compensation does not change a data file in any way. If as you are collecting a sample there are aberrations apparent in the data that might be related back to inaccurate compensation, immediately prepare a fresh set of compensation controls to re-run in the same session. Offline, you can remove any automatically assigned compensation to those samples and reapply a compensation matrix generated with the fresh, uncontaminated controls. However, the caveat is that in every way, the machine must be identical in set-up. Cytometers can shift in performance over time, so re-running comp controls must be done in the same session.
Supporting Your Research
Reducing spillover upfront during experiment design minimizes the amount of compensation needed later. FluoroFinder’s Panel Builder walks you through the panel design process to ensure you’re selecting fluorophores with minimal spectral overlap. With our premium subscription, you can access your panel’s and co-expression group’s complexity scores, another tool to reduce compensation.