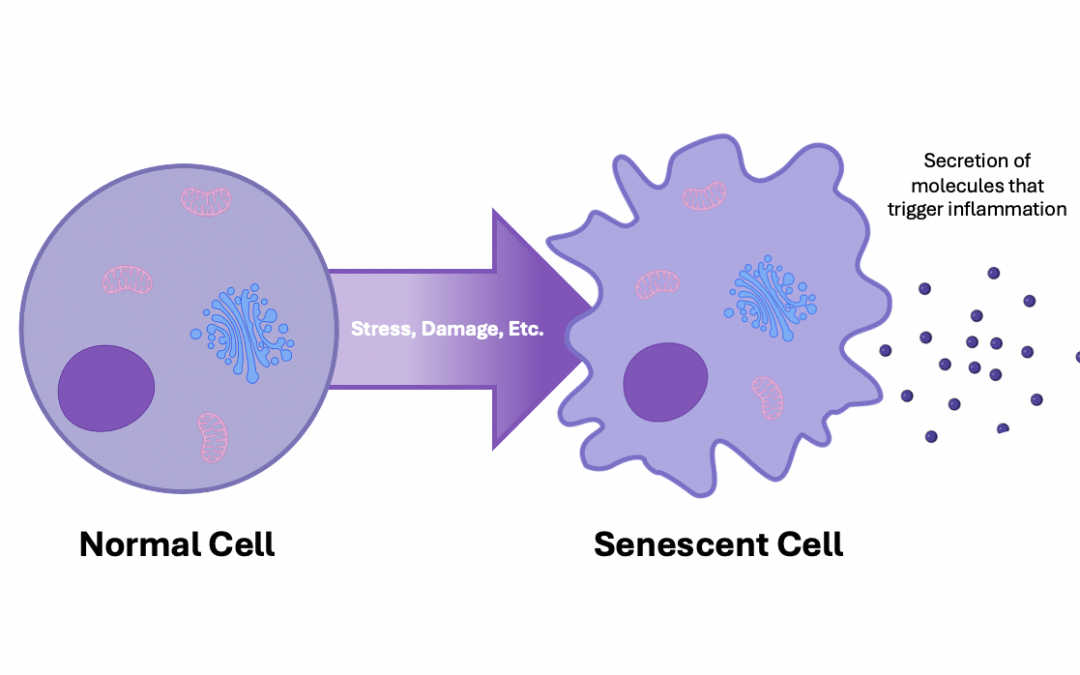
Cellular senescence has important roles in embryogenesis, tissue repair, and tumor suppression. However, prolonged senescence can be detrimental to human health, contributing to the development of conditions such as cancer and various age-related pathologies. Unraveling the mechanisms of senescence demands high-quality tools and technologies for investigating cellular phenotypes and functionalities. We spoke with Susan Keezer, Associate Director at Cell Signaling Technology (CST®) and Alessandro Serra, Field Applications Scientist at Cytek Biosciences, to learn about some of the options available to researchers.
Key Milestones in Senescence Research
Senescence was first described by Leonard Hayflick and Paul Moorhead in 1961, who observed that normal diploid cells in culture enter a permanent state of cell cycle arrest after a certain number of population doublings (1). Several decades later, Harley et al. linked this so-called ‘Hayflick limit’ to progressive telomere shortening, which is now a well-known hallmark of both senescence and organismal aging (2).
The first senescence biomarker was documented in 1995. Termed senescence-associated beta-galactosidase (SA-β-Gal), it demonstrated an age-dependent increase in skin cells, providing evidence that senescent cells accumulate with age in vivo (3). Other biomarkers of senescence have since been identified, including components of the senescence-associated secretory phenotype (SASP), a complex mixture of pro-inflammatory cytokines, chemokines, and other factors that exerts a broad range of autocrine and paracrine effects on the surrounding tissue microenvironment.
In 1997, Serrano et al. introduced the groundbreaking concept of oncogene-induced senescence, a sustained antiproliferative response prompted by aberrant activation of oncogene signaling (4). Senescence was subsequently found to be induced by many other stress signals, including telomere uncapping, DNA damage, and oxidative stress, reinforcing its essential protective role in limiting the proliferation of damaged and potentially tumorigenic cells.
More recently, senescence has been shown to be important for normal physiological processes such as embryogenesis, cutaneous wound healing, and tissue repair. By better understanding senescence, including how it influences aging at the organism level, researchers aim to develop effective therapies for conditions including cancer, diabetes, and neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases.
The Critical Importance of Multiplexed Detection
Because cellular senescence cannot be identified by a single biomarker, methods that allow for multiplexed detection are of critical importance for senescence research. “Flow cytometry is widely used for studying senescence as it lets researchers examine multiple markers in aggregate,” explains Keezer. “Besides SA-β-Gal activity, other established biomarkers of senescence include increased cell size and granularity, and altered expression of proteins such as p16 INK4A and p21 Waf1/Cip1. It is only by using combinations of different markers for characterizing senescent cells that researchers can have confidence in their results.”
When deciding which senescence markers to use, it is important to consider redundancy. “Most senescence biomarkers are shared with other aspects of cell biology,” cautions Serra. “For instance, cytoplasmic localization of p21 is also observed in cancer cells, where it is thought to act as a blocker of apoptosis. However, its correlation with low or negative levels of Ki67, a marker for ongoing cell division, should be indicative of cellular senescence rather than tumor. Similarly, several molecules composing the SASP, such as IL-6, IL-1 beta, MCP-1, have redundant biological functions. Thus, inferring senescence by sole detection of any of the SASP molecules would be difficult.”
If solid tissue samples will be used for studying senescence via immunohistochemical staining methods, researchers should be aware that SA-β-Gal activity can only be measured in cryopreserved material and not in formalin-fixed paraffin-embedded (FFPE) tissues. One workaround is to use Sudan Black B to stain for lipofuscin, an aggregate of oxidized proteins, lipids, and metals that accumulates in senescent cells and has been shown to correlate with SA-β-Gal positive tissue areas (5).
Challenges for Senescent Cell Sorting
The heterogeneity of senescent cell populations, both in terms of cell size and the extent to which different senescence markers are expressed by each cell, presents challenges for traditional flow cytometry-based cell sorting. Further complications are introduced by the presence of proliferative pre-senescent cells or those that have undergone senescence escape, as well as the need to use a sorter equipped with a non-standard nozzle. Yet, despite these hurdles, a flow cytometry-based method for sorting senescent cells has recently been reported (6).
By using a combination of size, granularity, and SA-β-Gal activity (detected with C12FDG, a fluorogenic substrate of β-galactosidase), researchers at the University of Lille have been able, for the first time, to sort senescent cells from proliferating cells. Specifically, by defining the C12FDGhigh cells before delineating the largest and most granular cells based on forward scatter (FSC) and side scatter (SSC) values for sorting with a 200 µm nozzle, it was possible to obtain different sub-populations. This study paves the way for including extra markers, provided they are amenable to fluorescent detection.
Advantages of imaging flow cytometry for studying senescence
Imaging flow cytometry combines the high event rate of traditional flow cytometry with the single-cell image acquisition capability of microscopy, giving it broad utility for many different research applications. The first imaging flow cytometer, the Amnis ImageStream® 100 system, was launched in 2004 and has since been followed by the next-generation Cytek® Amnis® FlowSight® and the Cytek® Amnis® ImageStream®X Mk II systems.
“Imaging flow cytometry offers many advantages over traditional flow cytometry for studying cellular senescence,” says Ferra. “First, it can easily distinguish senescent cells from multiplets of proliferating cells or from artifacts, either by conventional evaluation based on masks and features, or by using advanced machine learning tools available in the Amnis® AI image analysis software to classify cells and recognize undesirable objects (7).
Second, high-magnification imaging flow cytometry can resolve detailed intracellular aspects of senescence such as abnormal organelles, or topological accumulation of markers which are not possible for traditional cytometers to measure. This facilitates identification of senescent cells from primary samples where extreme augmentation of cellular size is not generally observed, contrary to in vitro models.
In addition, recent work has taken advantage of the unique capabilities of imaging flow cytometry to identify senescent cells with minimal manipulation of samples7. Here, researchers were able to extrapolate indices that allowed them to detect cell senescence in primary cells as well as in cell lines induced to senescence by different stimuli or pathological conditions.”
Supporting senescence research
Within recent years, therapies targeting senescence have been shown to extend the lifespan and reduce tissue injury in several animal models (8). However, translating these findings into human patients requires further breakthrough discoveries. For this reason, companies including CST and Cytek Biosciences remain focused on developing novel reagents and technologies to support senescence research.
Learn more about CST’s antibodies for senescence markers by visiting their interactive senescence signaling pathway. And, to find out how the range of Cytek® Amnis® imaging flow cytometers can benefit your research, visit https://cytekbio.com/pages/amnis. Then, streamline the design of your experiment with FluoroFinder’s suite of tools that includes our easy to use Panel Builder and Spectra Viewer.
- https://pubmed.ncbi.nlm.nih.gov/13905658/
- https://pubmed.ncbi.nlm.nih.gov/2342578/
- https://pubmed.ncbi.nlm.nih.gov/7568133/
- https://pubmed.ncbi.nlm.nih.gov/9054499/
- https://pubmed.ncbi.nlm.nih.gov/23449538/
- https://pubmed.ncbi.nlm.nih.gov/37056241/
- https://pubmed.ncbi.nlm.nih.gov/36010584/
- https://pubmed.ncbi.nlm.nih.gov/35922662/