Flow cytometry assays are integral to cell manufacturing, where they have an essential role in safeguarding product quality and function. However, the types of controls used for flow cytometric characterization are changing as researchers realize the advantages of cell mimics over traditional fluorescent beads or biologically-derived materials. We spoke with Annalise Barnette, Ph.D., Senior Manager, Global Marketing at Slingshot Biosciences, who explained how precision-engineered cell mimics serve as more reliable controls for modern cellular testing.
What is Cell Manufacturing?
The term cell manufacturing is generally used to refer to the production of cell therapies – advanced cell preparations that are transplanted into a patient for medical purposes. The cells can either be autologous, meaning they are derived from the individual in question, or allogeneic, meaning they are sourced from a donor. In either scenario, some form of modification is typically required for the cells to perform their intended function. The following are some leading examples of cell therapies:
Chimeric Antigen Receptor (CAR) T cell Therapy
CAR T-cell therapy is a type of immunotherapy that harnesses T cells for targeted tumor cell killing. It involves isolating the T cells from the blood and engineering them to express a CAR at the cell surface; the modified cells are then expanded in culture and infused into the patient. Each CAR has an extracellular antigen-binding domain, which is derived from a tumor-specific antibody single chain variable fragment (scFv), and an intracellular signaling domain, which promotes T cell activation in response to target binding. The US Food and Drug Administration (FDA) has approved six CAR T-cell therapies to date, four targeting CD19 to treat relapsed or refractory B cell cancers and two targeting the B cell maturation antigen (BCMA) to treat multiple myeloma1.
Stem Cell Therapy
Stem cells have the unique abilities to self-renew and differentiate into other cell types. As a result, they are being investigated for many different regenerative medicine applications, as well as for the treatment of autoimmune disease. Recent publications describe testing of human embryonic stem cell-derived retinal pigment cells to treat age-related macular degeneration; evaluation of human umbilical cord-derived mesenchymal stem cells to alleviate the symptoms of osteoarthritis; and assessment of intestinal epithelial stem cells to suppress inflammatory bowel disease2,3,4. Currently, the only stem cell products that are FDA-approved consist of hematopoietic progenitor cells that are derived from umbilical cord blood5.
Tumor-Infiltrating Lymphocyte (TIL) Therapy
TIL therapy involves isolating the natural infiltrating lymphocytes from a tumor, expanding them in vitro, and infusing them back into the patient with a high dose of interleukin-2 to stimulate TIL activity. Because TILs are composed of multiple clones, they have the potential to address challenges posed by tumor heterogeneity6. However, following FDA approval of the first TIL therapy in 2024 (lifileucel for adult patients with advanced or unresectable melanoma), the supporting clinical trials have identified possible adverse events that highlight the need for further development7.
The Importance of Flow Cytometry to Cell Manufacturing
Flow cytometry represents an essential technique for cell manufacturing, where it is used to monitor critical quality attributes (CQAs) throughout the entire product lifecycle. Applications of flow cytometry include confirming cellular phenotype and function, checking for contaminating cell types, and monitoring the surface expression of CARs. However, because flow cytometry currently lacks international reference standards, product developers must ensure that flow cytometry experiments are tightly controlled in order to generate reproducible and accurate data.
How do Cell Mimics Improve on Standard Controls for Flow Cytometry?
Standard controls for flow cytometry consist of either fluorescent beads or biologically-derived materials. A major advantage of fluorescent beads is that they represent a convenient, shelf-stable solution. However, this benefit is offset by the fact that fluorescent beads can never fully resemble biological samples. Live cells are more biologically relevant controls, comprising hugely complex systems. Yet live cells face stability, consistency, storage, and supply-chain issues, amongst other challenges. To overcome the various limitations of standard controls, there is an increasing drive towards the use of alternative solutions, such as cell mimics8. Not only are cell mimics more consistent than traditional controls, but they also have superior stability, are non-biohazardous and easily scalable, and allow for customization.
Tunable Cell Mimics from Slingshot Biosciences
Slingshot Biosciences’ cell mimics* allow for tuning of cellular characteristics such as granularity, size, refractive index (RI), surface biomarker expression, and fluorescence. In addition, it is possible to embed DNA or RNA in cell mimics and create controls for orthogonal validation of sequencing assays.
“Besides being highly consistent, our cell mimics can be manufactured at scale without sacrificing performance or reliability,” reports Barnette. “They are also incredibly easy to use – simply reconstitute the lyophilized product in PBS, perform any necessary staining, and run the flow cytometry assay as per usual.” Figure 1 shows a comparison of isolated white blood cells (WBCs) and Slingshot Biosciences’ FlowCytes® PBMC mimics, demonstrating true-to-life scattering and fluorescence.
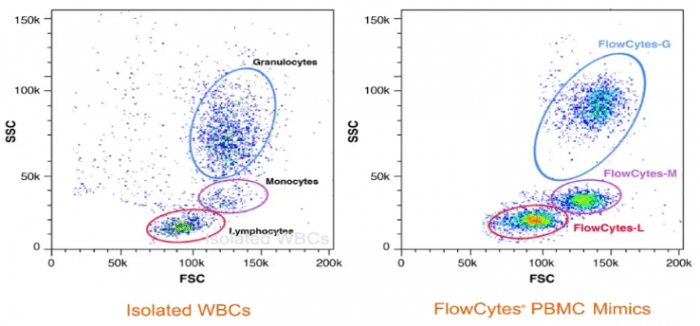
Figure 1. Comparison of isolated white blood cells (WBCs) and Slingshot Biosciences’ cell mimics.
Applications of Slingshot Biosciences’ Cell Mimics
Slingshot Biosciences’ cell mimics support a variety of functions. “Our FlowCytes® Non-Fixed White Blood Cells serve as a forward and side scatter control for instrument standardization,” explains Barnette. “In addition, we offer TruCytes™ Biomarker Controls, which can be custom designed to express specific markers.” Other Slingshot products include ViaComp® Cell Health Controls, which bind to DNA intercalating dyes and amine-reactive dyes to simulate viability staining, and SpectraComp® Compensation Controls, which are single stain controls that optimize spectral unmixing results to reduce errors associated with spectral overlap.
Slingshot Biosciences in the Cell Therapy Process
Cell mimics have broad utility for cell manufacturing applications, as shown in Figure 2. For example, TruCytes™ Lymphocytes Subsets Cell Mimics can be used for defining cell subsets in patient samples, while custom TruCytes Biomarker Controls and SpectraComp Compensation Controls facilitate applications such as detecting rare biomarkers, monitoring cell isolation, and measuring transduction efficiency. “We’ve also recently launched TruCytes Potency Cell Mimics to support CAR T-cell development for CD19- or BCMA-based CARs,” says Barnette. “In In this setting, cell mimics with specific markers, namely CD19 or BCMA, are used as target cells in co-culture for functional potency assessments. In response to CAR T-cell binding, interferon-γ is generated, which can be measured by ELISA or a cytometric bead array assay. Slingshot’s technology enables the incorporation of additional CAR targets beyond BCMA and CD19 to create customized potency cell mimics.”
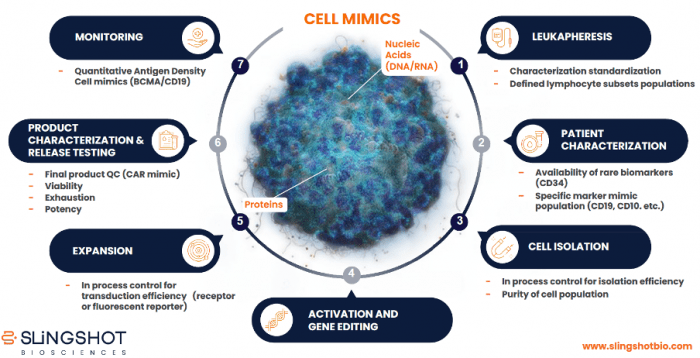
Figure 2. End-to-end applications of cell mimics across the cell therapy process.
Supporting Your Research
FluoroFinder has developed a suite of tools to help streamline your research. These include our Flow Cytometry Panel Design tool, which simplifies the process of selecting the best fluorophore combinations, and our Spectra Viewer, which lets you compare the spectral properties of more than 1,000 dyes alongside instrument-specific laser and filter configurations.
You might also want to refer to Slingshot’s extensive range of resources, which includes a case study reporting the use of custom TruCytes controls to validate a prognostic FcγRIIa flow cytometry test on fixed platelets and a recent Cytometry Part A publication that describes a reference control comparison.
*Slingshot Biosciences’ cell mimics are for research use only (RUO) and are not intended for use in clinical or diagnostic applications.
References:
- Parums DV. A Review of CAR T Cells and Adoptive T-Cell Therapies in Lymphoid and Solid Organ Malignancies. Med Sci Monit. 2025;31:e948125. doi:10.12659/MSM.948125
- Sharma A, Jaganathan BG. Stem Cell Therapy for Retinal Degeneration: The Evidence to Date. Biologics. 2021;15:299-306. doi:10.2147/BTT.S290331
- Lu JS, Song CY, Cheng WJ, Wang KY. Mechanisms and challenges of mesenchymal stem cells in the treatment of knee osteoarthritis. World J Stem Cells. 2025;17(4):102923. doi:10.4252/wjsc.v17.i4.102923
- Lin Q, Zhang S, Zhang J, et al. Colonic epithelial-derived FGF1 drives intestinal stem cell commitment toward goblet cells to suppress inflammatory bowel disease. Nat Commun. 2025;16(1):3264. doi:10.1038/s41467-025-58644-2
- https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/important-patient-and-consumer-information-about-regenerative-medicine-therapies
- Zhao Y, Deng J, Rao S, et al. Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges. Cancers (Basel). 2022;14(17):4160. doi:10.3390/cancers14174160
- Parums DV. A Review of CAR T Cells and Adoptive T-Cell Therapies in Lymphoid and Solid Organ Malignancies. Med Sci Monit. 2025;31:e948125. doi:10.12659/MSM.948125
- Salehi-Reyhani A, Ces O, Elani Y. Artificial cell mimics as simplified models for the study of cell biology. Exp Biol Med (Maywood). 2017;242(13):1309-1317. doi:10.1177/1535370217711441