Conjugating antibodies to other molecules is a common technique used for drug delivery, changing solubility, attaching to solid substrates (for purification or assays), and visualizing the antibody using fluorescent, magnetic or gold nanoparticles. Western blots, fluorescence microscopy and flow cytometry all depend on fluorescently tagged antibodies for visualization. Although fluorescently conjugated antibodies have been available for decades, it’s not always possible to find an off-the-shelf solution for your antigen of interest, so researchers often perform their own conjugation. Conjugating primary antibodies lets the researcher use the same isotype for different antigens in a single protocol- impossible when using labeled secondary antibodies.
Two main approaches for antibody labeling will be discussed in this article. The first involves direct bond formation between reactive amino acid sidechains and a modified fluorophore. This can yield high degrees of labeling (DOL > 5 fluorophores per antibody), but tends to have less specificity. It is important to characterize (DOL, antibody binding properties) and purify the resulting conjugates to verify performance. The second method involves specific labeling of the antibody’s Fc (constant) region with lower DOL (< 5 fluorophores per antibody). Kits are available to simplify both of these procedures, reduce purification steps and provide consistent results. Many companies also provide conjugation services, including Jackson ImmunoResearch Labs, BioLegend, Cell Signal, Fortis and others.
Amino Acid Labeling
Many amino acid side chains contain reactive groups such as thiol/ sulfhydryl, alcohol, carboxylic acids and amines. While there are techniques to conjugate to many of these (Kjaersgaard 2022), the simplest to conjugate are primary amines, the N-terminus of the protein and Lysine (Lys), and Cysteine (Cys), a thiol or sulfhydryl).
Lysine and Primary Amines
Lys is a very common amino acid (in the top 1/3 of abundance) with a primary amine sidechain. It has a pKa of ~10.5 and is mostly positively charged (protonated) at neutral pH. As such, it is preferentially positioned on the outside of a folded protein in aqueous solution, as is the protein’s N-terminus (Jacob 2007) which exhibits similar reactivity. The deprotonated form is one of the most nucleophilic amino acid sidechains. Conjugation reactions proceed by nucleophilic attack of the neutral primary amine of Lys on the carbon atom of isothiocyanate (NCS) or N-hydroxysuccinimide (NHS) ester (Figure 1). For NCS, this yields a substituted thiourea (Figure 1a), and for NHS, it yields an amide bond and the NHS leaving group (Figure 1b).
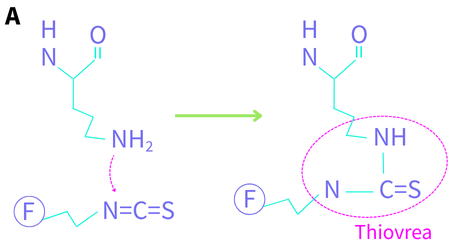
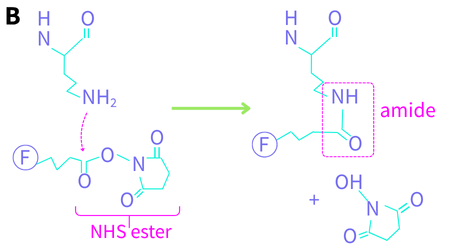
Figure 1. Lysine conjugation chemistry. The protein’s peptide backbone is shown at the top. F denotes a fluorophore. (A) the nitrogen lone pair attacks the carbon of the isothiocyanate to yield a thiourea (B) the nitrogen lone pair attacks the carbonyl carbon of the NHS ester to yield an amide.
Fluorescein isothiocyanate (FITC), the best-known NCS reagent, has been available since the early 1960s as fluorescence labelling techniques were starting to become mainstream. Other fluorophores available with this reactive sidechain include Rhodamine B, Texas Red and several cyanine dyes. The Lys-NCS antibody conjugation reaction occurs at pH 9-10 over several hours (Figure 1a).
At elevated temperatures, the FITC-conjugated antibody is unstable (Banks 1995), which led to the development of NHS esters in the early 1980s. NHS-ester-fluorophores and kits are available from a large number of manufacturers with a wide array of fluorophores, from the UV and visible through the NIR (for small animal labeling). This conjugation reaction is performed at a milder pH (7-9) and is significantly faster than the NCS reaction, which is why it has largely taken over as the preferred amine-conjugation protocol. The pH dependence of the reaction can be exploited to selectively label the N-terminus (pKa ~ 8.9) at pH 6.5 (Selo 1996). To address the low solubility of NHS-dye complexes, negatively charged sulfo-NHS-dye complexes were developed. The reaction proceeds the same way with both forms of NHS.
Lysine-labelling is effective but there are several things to keep in mind:
- The specificity of this reaction is low, so antibodies should be purified before the reaction. Buffers must be free of other agents that can cross-react, including amines, azides, glycerol, BSA, etc.
- The results are heterogeneous – there is little to no control over the number or locations of labelled Lys residues.
- Fluorescent NCS and NHS reagents have poor aqueous stability. They are typically stored as powders or in dry DMSO/DMF at low temperatures to minimize hydrolysis. The powder or solution should be warmed to room temperature before opening to reduce water condensation.
- Cleanup steps are required to separate labeled antibodies from free dye. These steps can cause loss of precious labeled conjugates – impractical for very small reactions.
Cysteine (Cys)
Free Cys, much less common than Lys (in bottom 1/3 by count) and often covalently bound in disulfide bonds, is the most nucleophilic amino acid when deprotonated, with a pKa around 8.4. It reacts to form relatively unstable conjugates with both the NCS and NHS-esters described above. To increase the stability of the conjugates, maleimide chemistry was developed as an alternative, using a number of maleimide derivatives (Christie 2015).
This coupling proceeds through a Michael addition reaction, as shown in Figure 2. The second hydrolysis step provides stability to the resulting conjugate (Tumey 2014). Unlike Lys, which exhibits similar reactivity regardless of position, the reactivity of Cys with maleimide fluorophores is position-dependent and can be directed by adjusting the reaction conditions (Boll 2022).
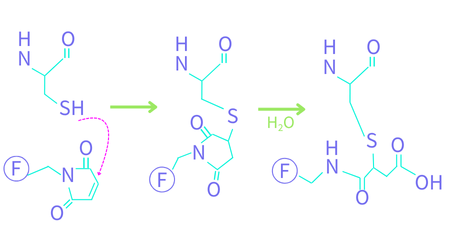
Figure 2. Fluorescent maleimide reaction with Cys. The protein’s peptide backbone is shown at the top. F denotes a fluorophore. The first reaction is a Michael addition followed by a ring-opening hydrolysis step.
Like the Lys-reactions, Cys-coupling reactions need to be cleaned up to remove unreacted reagents when the reactions are complete. A number of manufacturers offer fluorescent maleimide reagents, including Biotium, Lumiprobe, and ThermoFisher.
No-Cleanup Covalent Conjugation Kits
New single-reaction sized covalent labeling kits have been developed by Biotium and Abcam to eliminate the post-conjugation cleanup steps. The labels in these kits are stored and shipped in a solid form designed to react with a predetermined quantity of antibody in a single reaction.
Mix-n-StainTM kits from Biotium have been uniquely formulated to tolerate low concentrations of amines (<20mM Tris) and other interfering compounds. Reaction buffer is added to the antibody, the solution is transferred to the vial containing lyophilized fluorophore, labeling is completed in 15 minutes in the dark, then a storage buffer is added. A concentration and purification step can be performed before or after labelling if required. Kits are available for both IgG and IgM isotypes with over 30 CF dye options.
The Lightning Link kit (Abcam) 3-step conjugation kit is comprised of a buffer, lyophilized label mixture and quencher. The concentration of the lyophilized label mixture has been optimized so that there are only low levels of free label remaining at the end of the reaction. Any unreacted, unhydrolyzed label is quenched with a small water-soluble primary amine and washed out during wash steps of the subsequent staining protocol. This kit provides fast, reproducible conjugation. AbCam also sells purification kits to remove albumin, BSA and other confounding proteins from the antibody solution before using this kit.
Fc Specific Labeling
This method labels the Fc (constant) region of the antibody (Figure 3) to eliminate interference of conjugated amino acids with antigen binding in the Fab regions. These methods are not sensitive to buffer type or the presence of other proteins in solution. While they are more specific, the DOL tends to be lower (but less variable) using these methods.
Figure 3. Antibody illustration with Fc region noted.
Over 20 years ago, Molecular Probes (now ThermoFisher) developed Zenon technology, which uses fluorescently labeled antibody fragments against the Fc region of IgGs. Conceptually, this is similar to using fluorescent secondary antibodies. The reagents are specific to the organism (mouse, rabbit, human, goat) and are available with a number of fluorophore tags. At elevated temperatures, the conjugate is unstable which led to the development of newer approaches.
Proteintech’s FlexAble product labels the Fc region of the antibody with a fluorescently labeled polypeptide (FlexLinker) to bind the isotype-specific Fc region of select species (rabbit, mouse, rat, human) in minutes with high affinity. Excess FlexLinker is reacted with FlexQuencher (an IgG or Fc fragment) and washed away during wash steps of the staining protocol. FlexAble conjugation results in 2 fluorophores per antibody, Flexable 2.0 results in 5 per antibody. Both are available with a number of CoraLite and CoraLite Plus dyes.
The oYo-Link method, by AlphaThera, utilizes a genetically engineered protein derived from the Fc binding domain of staphylococcal protein A. The protein contains photoactive benzoylphenylalanine in the binding site, which crosslinks with the antibody backbone upon irradiation at 365 nm, forming a permanent covalent linkage at a designated position. This has been verified in antibodies from a number of species. The oYo-Link reagents are available with allophycocyanin and phycoerythrin fluorescent probes. Up to 2 fluorophores can be bound to the antibody with this method.
Supporting Your Research
There are many strategies for antibody conjugation available to the modern biologist. For high DOL requirements, amino acid conjugation reagents and single reaction kits are available. For more specific binding, with lower DOL and no change to Fab binding regions, both affinity and covalent Fc conjugation options are available.
Whether you’re using off-the-shelf conjugates or planning custom labeling strategies, FluoroFinder helps you streamline your workflow so you can focus on what matters most – your research. Use our Antibody Search to find fluorophores and dyes, or unconjugated antibodies with conjugation kit options. Put it together with our Spectra Viewer to visualize spectral overlap and optimize your marker-fluorophore combinations.
References:
- Banks, P.R. and Paquette, D.M. “Comparison of three common amine reactive fluorescent probes used for conjugation to biomolecules by capillary zone electrophoresis.” Bioconjugate Chem 6, no. 4 (July 1995): 447-458.
- Boll, L.B. and Raines, R.T. “Context-dependence of the reactivity of cysteine and lysine residues.” CHembiochem 23, no. 14 (2022): e202200258.
- Christie, R.J., et al. “Stabilization of cysteine-linked antibody drug conjugates with N-aryl maleimides.” J. Controlled Release 220 (2015): 660-670.
- Jacob, E. and Unger, R. “A tale of two tails: why are terminal residues of proteins exposed?” Bioinformatics 23, no. 2 (Jan 2007): e225-e230.
- Kjaersgaard, N.L., et al. “Chemical conjugation to less targeted proteinogenic amino acids.” ChemBioChem 23, no. 19 (July 2022): e202200245.
- Selo, I., et al. Preferential labeling of alpha-amino N-terminal groups in peptides by biotin: application to the detection of specific anti-peptide antibodies by enzyme immunoassays.” J. Immunol Methods 199, no. 2 (Dec 1996): 127-38.
- Tumey, L.N., et al. “Mild method for succinimide hydrolysis on ADCs: Impact on ADC potency, stability, exposure and efficacy.” Bioconjugate Chemistry 25, no. 10 (2014): 1871-1880.