Fluorescence-activated cell sorting (FACS) is an advanced form of flow cytometry that allows researchers to isolate specific cell types from a heterogeneous sample. This article explains the basic principles of FACS and describes some common research applications. It also highlights several leading FACS instruments, including their key features and benefits.
A Brief History of FACS
Cell sorting technology originated in 1965, when Mack Fulwyler created the first device capable of separating biological cells by volume. By modifying a Coulter counter with a newly developed ‘ink jet’ technique for making and charging liquid droplets, Fulwyler was able to isolate a small population of blood cells from a heterogeneous sample1,2.
Fulwyler’s method was subsequently adapted by Len Herzenberg and a team of engineers at Stanford University to separate cells based on fluorescence3. Through the introduction of a laser, it was possible to sort mouse spleen cells from Chinese hamster ovary (CHO) cells following the development of fluorochromasia.
Realizing the vast potential of this approach, Becton Dickinson (BD) licensed Stanford University’s technology and launched the first commercial fluorescence-activated cell sorter, the BD FACS™ II, in 1974. Since then, cell sorters have evolved to become faster, gentler, and capable of detecting more parameters. Although BD still owns the trade name FACS, the term is generally accepted to mean any fluorescence-based cell sorter, irrespective of vendor.
Principles of FACS
FACS instruments work on the same principles as flow cytometers, which combine fluidics, optics, and electronics to identify specific cell types within a heterogeneous sample. Common sample types for both platforms include whole blood, excised tissue, and cell cultures, all of which must be processed into a single-cell suspension and stained with fluorescently-labeled antibodies for specific markers prior to sorting.
Once the immunostained sample has been introduced into the FACS instrument, the fluidics system directs the cells in single file past an interrogation point, where multiple laser beams intercept. When a cell crosses the interrogation point, it generates scattered light and fluorescence signals that are guided toward specialized detectors. These convert the signals they receive into electrical currents, known as events, each corresponding to an individual particle. By gathering data from many thousands of events, the flow cytometer provides a graphical interpretation of the sample.
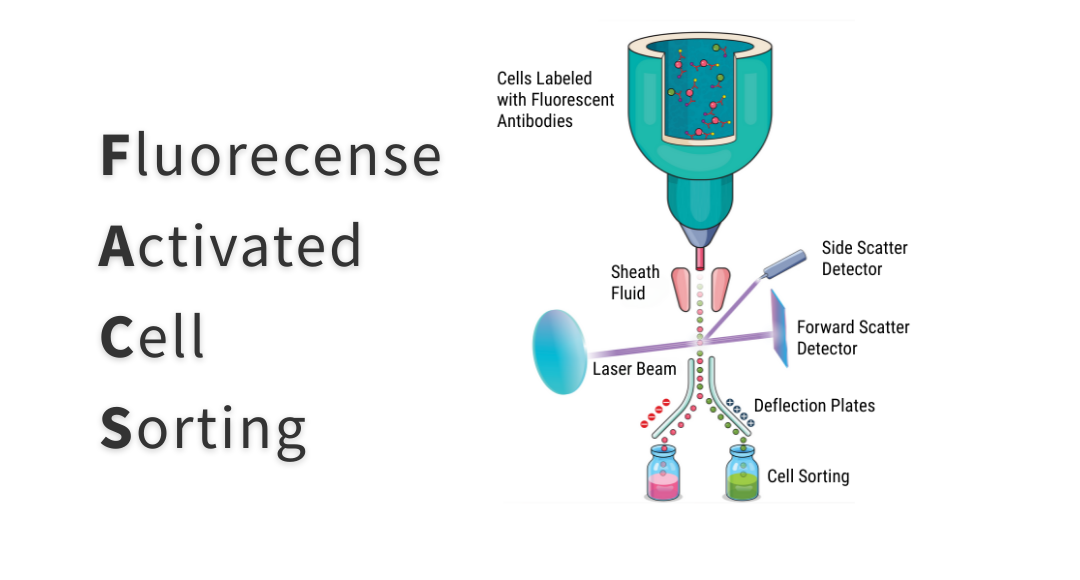
The main difference between flow cytometers and FACS instruments is that the latter enable researchers to retain cells of interest for further use. To achieve this, traditional FACS instruments vibrate the flow stream at a high frequency to form droplets and apply an electric charge to any droplets containing target cells. As the droplets leave the interrogation point, they enter an electrostatic field, where they are deflected into collection tubes, as shown in Figure 1.
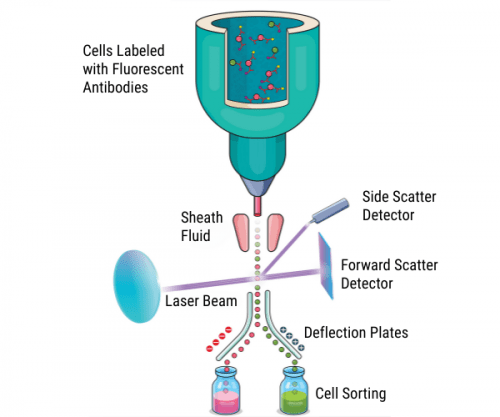
Figure 1. Principles of traditional fluorescence-activated cell sorting.
FACS Protocol
A typical FACS protocol begins with generating a single-cell suspension and performing a cell count, which usually includes an assessment of cellular viability. The desired number of cells is then pelleted by centrifugation, washed several times, and resuspended in a blocking buffer.
After blocking, the cells are incubated with one or more primary antibodies. Often, these are labeled with fluorescent dyes to allow for direct detection. Alternatively, unlabeled primary antibodies may be used, meaning that fluorescently-labeled secondary antibodies are required for indirect detection.
Once the immunostaining process is complete, the cells are washed to remove any unbound antibodies before being resuspended in a suitable buffer for cell sorting. Critically, every FACS protocol should be carefully optimized to ensure accurate, reproducible results.
3 tips for performing FACS experiments
- When working with whole blood, lysing red blood cells prior to immunostaining is important to properly gate leukocytes
- For many sample types, an Fc blocking step is recommended to prevent antibodies from binding non-specifically to Fc receptors on the surface of immune cells
- The use of a cell-permeant viability dye, such as propidium iodide (PI), DAPI, or 7-AAD, is advised to exclude dead cells from the analysis
FACS Research Applications
One of the best-known applications of FACS is its use to unravel the complexities of the immune system. By isolating distinct populations of immune cells, researchers can better understand which cell types are involved in processes such as pathogen defense, stimulating immune responses to vaccines, and producing auto-antibodies in conditions such as systemic lupus erythematosus (SLE).
FACS is also important for cancer research, where its uses include identifying cancer biomarkers to develop targeted therapies, isolating tumor infiltrating immune cells to learn how tumors evade immune detection, and analyzing cells that survive chemotherapy to investigate the mechanisms behind drug resistance.
Other fields that depend on FACS include stem cell research, where isolating hematopoietic stem cells allows for exploring the differentiation pathways that lead to various types of blood cells, and monoclonal antibody discovery, where FACS is widely used for single B cell screening. Additionally, FACS has utility in clinical settings to monitor cellular responses to therapies.
FACS Instrumentation
In the 50+ years that have passed since commercialization of the BD FACS II, BD has continually advanced its instrumentation. While all current systems retain the core principles of droplet formation and electrostatic deflection via polarized plates, two transformative developments have redefined performance: the introduction of flow cells for enhanced signal sensitivity and streamlined workflows, and the refinement of optical systems and detectors to enable multi-parametric analysis for targeted cell sorting.
BD’s cell sorting portfolio now spans four product families, each leveraging one of three optical and detection methodologies. The BD FACSMelody™ and BD FACSAria™ models (BD FACSAria™ III and BD FACSAria™ Fusion) utilize conventional polychromatic flow cytometry, which supports up to 20 detectors (18 colors) with five lasers and four-way sorting at up to 25,000 events per second (e/s).
The BD FACSymphony™ S6 extends capabilities to six-way sorting at 25,000 e/s, offering 50 detectors across up to nine lasers for simultaneous analysis of 28 colors (with conventional PMTs and compensation), or more using a spectral-enabled workflow (48 PMTs and spectral unmixing).
The BD FACSDiscover™ S8 integrates spectral flow cytometry with real-time imaging, employing up to 86 detectors – including APDs, PMTs, and photodiodes – across five lasers to accommodate higher-dimensional panels (proven for up to 50 colors4) alongside 78 image-derived features that capture spatial localization of signals. This integration enables unprecedented analytical depth, combining spectral flow cytometry, cellular morphology, and spatial insights to facilitate subcellular classification through analysis and sorting.

Figure 2. The BD FACSDiscover™ S8.
CytoFLEX SRT Benchtop Cell Sorter – Beckman Coulter Life Sciences
The CytoFLEX SRT Benchtop Cell Sorter* for research from Beckman Coulter Life Sciences is capable of meeting requirements for a wide range of sorting needs. Like all CytoFLEX instruments, it includes innovative technologies that simplify instrument setup and operation, empowering investigators to focus on their research. The Violet-Blue-Yellow Green-Red (V-B-Y-G-R) Series has 15 fluorescent detectors when fully activated. However, it can be purchased with as few as five, with an option to activate additional lasers and detectors using an activation key. The CytoFLEX SRT is capable of complex sort logic, including 4-way sorting and Mixed Mode sorting, and also has the ability to catch aborts and preserve precious cells.
* For Research Use Only. Not for use in diagnostic procedures.
 Figure 3.The CytoFLEX SRT Benchtop Cell Sorter.
Figure 3.The CytoFLEX SRT Benchtop Cell Sorter.
Invitrogen™ Bigfoot™ Spectral Cell Sorter – Thermo Fisher Scientific
The Invitrogen™ Bigfoot™ Spectral Cell Sorter is Thermo Fisher Scientific’s flagship instrument, configurable with up to 9 lasers and 60 detectors. Capable of 6-way sorting into tubes, 4-way sorting into 96-well and 384-well plates, or straight-down sorting into 1536-well plates, it can sort as many as 70,000 e/s, depending on application.
The Bigfoot Spectral Cell Sorter is high-speed and high-throughput, capable of sorting a 96-well plate in as little as 11 seconds and a 384-well plate in 20 seconds. Simple enough for an individual lab, and robust enough for a core facility, the Bigfoot Spectral Cell Sorter can help you master the full range of sorting experiments such as deep immunophenotyping, genomics, cell and gene therapy research, single-cell sorting, and other high-performance, high-throughput cell sorting applications.
All Bigfoot Spectral Cell Sorter configurations enable traditional compensation methods to account for spillover from one fluorophore into another fluorophore’s detector. In addition, most Bigfoot configurations have spectral flow cytometry capability, allowing for either spectral unmixing or traditional compensation to separate the signals from different fluorophores. With Bigfoot spectral configurations, you can run both conventional and spectral experiments on the same sorter and convert experiments from traditional compensation to spectral when you need more parameters.
Other key features include automated stream calibration, quality control, and drop delay, which help to reduce user errors, and integrated biocontainment with access to a built-in sample vortex mixer, tube rack, and biohazard bag, which enables the operator to complete many routine tasks without breaching the safety barrier.
Available configurations include the following:
Non-Spectral Instruments:
Spectral Instruments:
- Bigfoot™ Spectral Cell Sorter, 9 lasers, 60 parameters
- Bigfoot™ Spectral Cell Sorter, 7 lasers, 60 parameters
- Bigfoot™ Spectral Cell Sorter, 5 lasers, 53 parameters
 Figure 4.The Invitrogen™ Bigfoot™ Spectral Cell Sorter.
Figure 4.The Invitrogen™ Bigfoot™ Spectral Cell Sorter.
Supporting Your Research
Whatever type of FACS experiment you’re performing, FluoroFinder can help! Use our Antibody Search function to find antibodies that are validated for flow cytometry, then turn to our Spectra Viewer to check which dyes are compatible with your FACS instrument. And, for a detailed look at the optical properties and spectral profiles of different dyes, check out our Fluorescent Dye Database for detailed information on more than a thousand different fluorochromes.
References:
- Fulwyler MJ. Electronic separation of biological cells by volume. Science. 1965;150(3698):910-911.
- Sweet RG. High Frequency Recording with Electrostatically Deflected Ink Jets. Rev. Sci. Instrum. 1965;36, 131–136.
- Hulett HR, Bonner WA, Barrett J, Herzenberg LA. Cell sorting: automated separation of mammalian cells as a function of intracellular fluorescence. Science. 1969;166(3906):747-749.
- Konecny AJ, Mage PL, Tyznik AJ, Prlic M, Mair F. OMIP-102: 50-color phenotyping of the human immune system with in-depth assessment of T cells and dendritic cells. Cytometry A. 2024;105(6):430-436. doi:10.1002/cyto.a.24841