Recombinant antibody technology is addressing known limitations of traditional antibody molecules and opening up new areas of scientific research. We spoke with Michael Fiebig, Ph.D., Chief Scientific Officer at Absolute Antibody (a Vector Laboratories Company), Mary Mills, M.S., Product Manager, ZooMAb® Recombinant Antibodies at MilliporeSigma (the U.S. and Canada Life Science business of Merck KGaA, Darmstadt, Germany), and Michael Lyons, Ph.D., Product Manager, Recombinant Antibodies at Thermo Fisher Scientific to learn more about how recombinant antibodies are produced and the advantages they offer, as well as discuss state-of-the-art solutions for developing recombinant antibody products.
Methods for Producing Recombinant Antibodies
In general, there are three main routes for producing recombinant antibodies. First, an existing antibody sequence may be cloned into an expression vector, which is then introduced into host cells for in vitro antibody synthesis. Second, antibody display methods (e.g., phage, yeast, mammalian, or ribosome display) allow for identifying high-performing antibodies from a heterogeneous pool based on binding to an immobilized target. Third, single B cell screening technologies are used to isolate antigen-specific B cells from blood for screening and subsequent sequencing of optimal antibody clones.
“At Absolute Antibody, we sequence existing hybridomas, or use sequences derived via other methods, to produce antibodies in mammalian cells – typically HEK293, but occasionally CHO cells,” explains Fiebig. “We deliberately opted against doing discovery ourselves as there is a wealth of established antibodies that have formed the backbone of decades of research. I believe that where antibodies already exist, we have an obligation to produce them recombinantly and assess their performance first, before deciding if new clones should be generated. This is to avoid creating a new angle to the reproducibility crisis where established methods using a specific clone cannot be accurately replicated due to a lack of availability of high quality material.”
MilliporeSigma produces its ZooMAb® recombinant antibodies using a proprietary B cell immortalization technology, which enables a high-yield recombinant expression system and facilitates the generation of a broad range of clones; these are then screened to identify the best-performing antibodies for further development. “Many of our ZooMAb recombinant antibodies are rabbit monoclonals since, compared to commonly used murine species, rabbits recognize a greater diversity of epitopes per antigen and produce a higher immune response to ‘difficult’ immunogens such as small molecules, peptides, and post-translationally modified proteins,” says Mills. “Importantly, ZooMAb recombinant monoclonal antibodies are developed with a sustainable eco-design approach, aimed to minimize the environmental impact.”
“ThermoFisher is pursuing multiple strategies to generate an expansive portfolio of recombinant antibodies,” reports Lyons. “These include converting existing hybridoma monoclonal antibodies into a recombinant format by sequencing high-performance clones, utilizing phage display libraries, and leveraging advanced technologies such as our Bigfoot Spectral Cell Sorter to perform large scale single B cell sorting on immunized animal models and identify favorable antibodies for recombinant expression. Additionally, we are using known antibody sequences to produce recombinant multiclonal antibody cocktails, called Superclonal Recombinant Antibodies. These provide the precision of recombinant antibodies with the benefit of multi-epitope recognition seen in polyclonal antibodies.”
Advantages of Recombinant Antibodies
Recombinant antibodies offer many advantages over traditional polyclonal and hybridoma-derived monoclonal antibodies. These include exceptional batch-to-batch reproducibility, which eliminates issues related to cell-line drift and mutations commonly observed in hybridoma production. Recombinant antibodies also exhibit enhanced specificity and sensitivity, enabling precise detection of target proteins in complex biological samples. Furthermore, because recombinant antibodies are generated from defined sequences, under tighter control compared to extracting antibodies from animal sources, their use can provide more reliable and reproducible results, as well as can mitigate against accidental hybridoma loss due to contamination or a freezer malfunction.
Another key benefit of recombinant antibodies is that they reduce animal use for research. Once the antibody sequence is obtained, production can be achieved using animal-free methods, with no need for animal-derived components (e.g., serum). Additionally, recombinant antibodies can enable faster production, although Fiebig cautions that while methods such as phage display take just a few weeks for selection, researchers should factor in extra time for antibody production and re-validation. Importantly, many further advantages can be gained as a result of recombinant antibodies’ vast capacity for engineering.
Recombinant Antibody Engineering
Recombinant antibody engineering has increased flexibility for established research applications, such as flow cytometry and IHC, as well as paved the way for developing novel techniques, including those based on the use of multi-specific antibodies. One of the best known approaches, species switching, involves changing the antibody backbone to that of a different animal. It can increase diversity in a panel of antibodies for multiplexing or be used to reduce immunogenicity for in vivo applications. Another popular strategy, class switching, alters an antibody’s isotype or subtype for reasons that include increasing avidity and improving in vivo effector function and stability.
Engineering can also be used to generate antibody fragments, such as Fab and scFv. “The smaller size of antibody fragments compared to their full-length counterparts lets them penetrate tissues more easily and clear more readily from animal systems through the renal pathway,” comments Mills. Antibody fragments also provide a means of abolishing Fc interference (the unwanted interaction between antibodies and Fc receptors expressed on the surface of immune cells), which may alternatively be addressed by introducing point mutations into the Fc region. “Our Fc Silent™ Antibodies contain key point mutations that abrogate Fc receptor binding, enabling researchers to reduce non-specific background in immunostaining methods and remove the antibody effector function in vivo,” says Fiebig.
Lyons adds that modifying the Fc region is a powerful tool Thermo uses to enhance antibody performance. Figure 1 shows testing data in immunocytochemistry for Thermo Fisher Scientific’s Parkin (A) and OCT4 (B) wild type and recombinant rabbit monoclonal antibodies, with the engineered products both demonstrating superior sensitivity over the wild type equivalents.
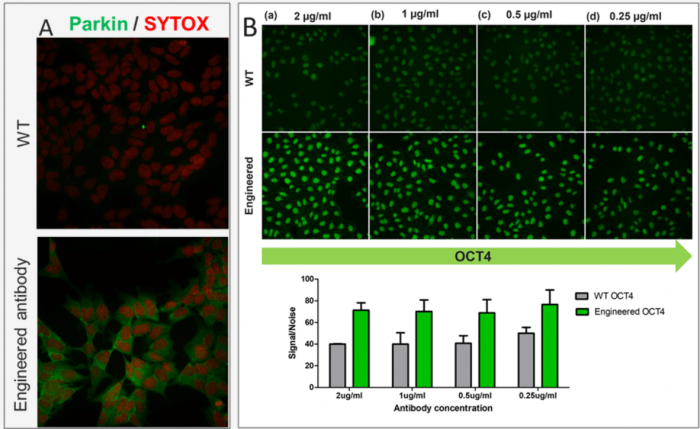
Figure 1. Testing data for Parkin (A) and OCT4 (B) recombinant rabbit monoclonal antibodies. Immunocytochemistry analysis of Parkin and OCT4 was performed using 70% confluent SH-SY5Y and NTERA-2 cells, respectively. The cells were probed with wild type (WT) and engineered Parkin or OCT4 Recombinant Rabbit Monoclonal Antibody at the indicated concentrations (Product # 740019R or 740020R). For Parkin, cells were also stained for nuclei, using SYTOX™ Deep Red Nucleic Acid Stain (Product # S11381).
Other engineering strategies include the addition of tags, which can aid purification, detection, immobilization, and functionalization, and the process of humanization. “While humanization is commonly used to reduce immunogenicity, we have at times also applied our Prometheus™ humanization platform to overcome manufacturability and stability issues with research antibodies,” reports Fiebig. “This is not because the antibody needed to be human, but because many of the physical requirements of a humanized antibody in the clinic align with a good research tool antibody: reliable manufacture, low aggregation, high stability, and consistent performance.”
Future Perspectives
Demand for recombinant antibodies continues to rise, driven by factors including their superior quality, capacity for tailoring to specific targets or applications, and the reduced animal burden associated with their production. “Researchers are showing strong preference for our recombinant antibodies” says Lyons. “The benefits of recombinant antibody technology, combined with our advanced validation processes, provide a valuable assurance in the reliability and accuracy of our antibodies, which translates to more confidence in experimental results.”
Echoing these sentiments, Mills notes that the shift towards recombinant antibodies demonstrates a commitment to advancing research and therapeutic options in a more reliable and effective manner. “Recombinant antibody availability has significantly transformed the landscape of biomedical research and therapeutic development,” she says. “Today, recombinant antibodies are integral to drug development, diagnostics, and therapeutic interventions that are paving the way for more precise and effective treatments across a range of diseases.”
Supporting Your Research
If you’re looking for recombinant antibodies to advance your research, FluoroFinder can help! Our Antibody Search function enables you to quickly and easily find products that are validated for your chosen application. And, for fluorescence-based applications, our Spectra Viewer lets you to compare the spectral properties of more than 1,000 dyes alongside instrument-specific laser and filter configurations, simplifying the process of experimental design.