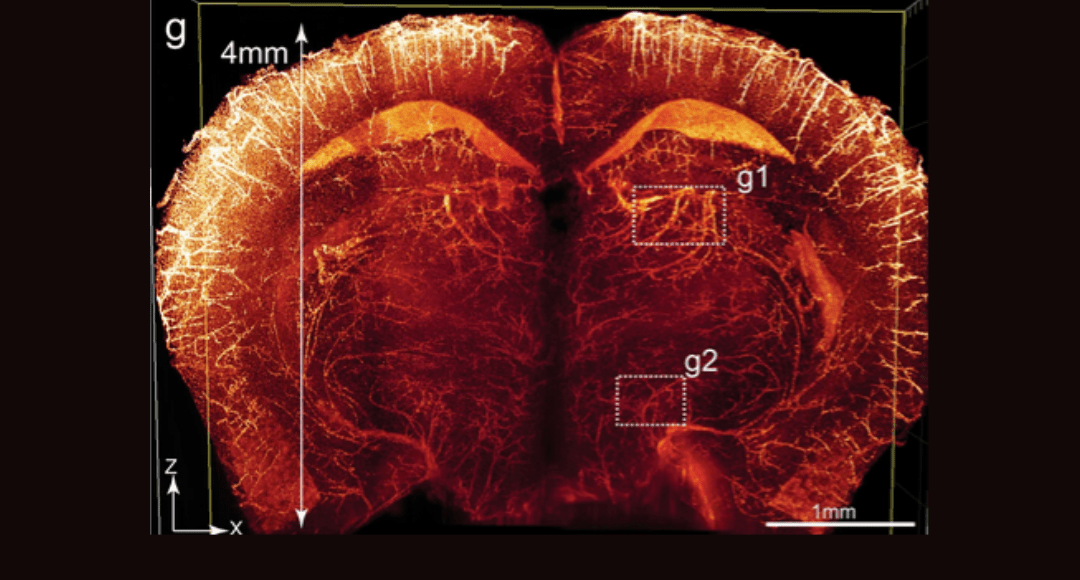
Cover Image: Imaging of the whole-brain vasculatures at sub-cellular resolution. Brains of adult Tie2-Cre;tTAflox;tetO-H2BGFP (TTH) mice were cleared using the PEGASOS method and imaged with a tiling light-sheet microscope. g An X-Z optical slice acquired at the hippocampus position was displayed. Used without modification under Creative Commons Attribution 4.0 International License from (Jing, 2018) Figure 7.
With the advent of 3-D fluorescence imaging methods including confocal, multiphoton and light-sheet microscopy, the workflow for preparing thick samples has been greatly simplified. Researchers no longer have to physically cut thick specimens into thin (< 10 micron) slices, individually stain and image those slices and align and reconstruct the images into a 3-D dataset. Now, a thick specimen can be imaged in its entirety – limited only by the working distance and resolution of the objective lenses and the ability for photons to reach and return from the focal plane. Scattering (or cloudiness) is a major limitation to achieving this goal, especially in samples > 1 mm thick.
Scattering in a sample comes from light refraction and reflection at the boundaries of different refractive index (n) materials. The larger the difference in n, the greater the potential for scattering. In tissues, there are many such material boundaries, as shown in Table 1. Mie scattering dominates most biological tissues, where the size of scattering elements are ~ 1/10th to several times the wavelength (the size of most organelles). Raleigh scattering for smaller features (e.g. individual proteins) at lower wavelengths and absorption also limit imaging depth.
Table 1. Refractive indices of some common biomolecules.

“Clearing” is the process of maximizing the transmission of photons through a sample, making it transparent. Clearing protocols remove the higher and/or lower n materials and attempt to index match the remainder to reduce or eliminate n-interfaces. There are two basic approaches to clearing – dehydration and aqueous (hydrophilic). In this article, we describe solvent dehydration of the sample followed by index matching with high-n organic compounds. This protocol has been around in one form or another for over 100 years (originally as part of the paraffin embedding process). Next month, we will discuss the hydrophilic methods, including those that use hydrogel materials.
Solvent-Based (Dehydration) Methods
The general procedure for these methods is:
- Tissue fixation
- Optional decalcification with EDTA solution
- Optional decolorization
- Optional IHC labeling
- Dehydration via concentration gradient washes
- Optional IHC labeling (after rehydration washes, followed by a second dehydration process)
- Optional delipidation
- Index-matching and imaging
For tissue slices and small whole mounts, the above protocol is accomplished through passive diffusion or gentle stirring. These include the BABB (Foster, 2019) and DISCO (Molbay, 2021) methods and commercial clearing kits from Tocris and Visikol. For larger whole mounts and whole animals, perfusion methods are used to deliver reagents using the existing circulatory system. PEGASOS, (Jing, 2018) iSHANEL (Zeng, 2025) and uDISCO (Molbay, 2021) have been used to fix and clear entire animals.
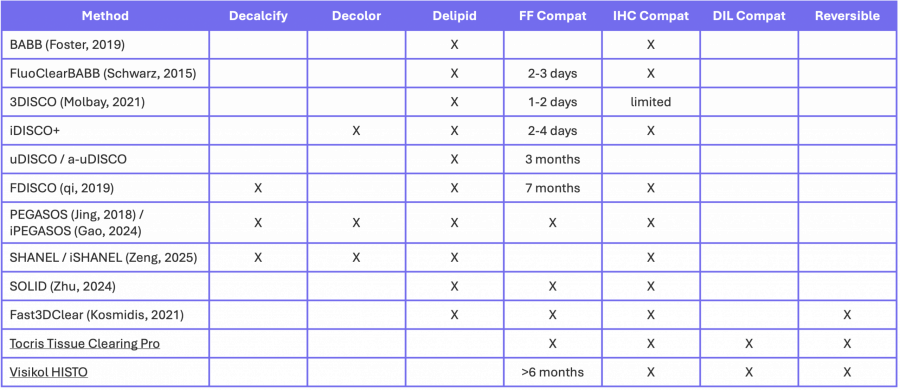
The first 3 columns of Table 2 summarize the optional steps in a number of protocols. Note that that is not a comprehensive list of protocols since tissue clearing is an active area of research.
Table 2. Optional steps and types of labelling that can be completed for solvent-based clearing.
Decalcification
Especially in whole animal imaging studies, the presence of bone, exoskeletons and teeth obscure many structures. Perfusing the animal with a relatively concentrated EDTA solution reduces bony tissues to protein scaffolds.
Decolorization
Removal of heme and other chromophores often required for whole-organ (especially liver and kidney) and whole-animal imaging. A number of amino alcohols have been used (Figure 1) including solutions of Quadrol in PEGASOS, and NMDEA in iSHANEL. Hydrogen peroxide can also be used for decolorization as in iDISCO. In Figure 2, the importance of decolorization is evident by comparing results using uDISCO (no decolorization) and PEGASOS methods. (Jing, 2018)
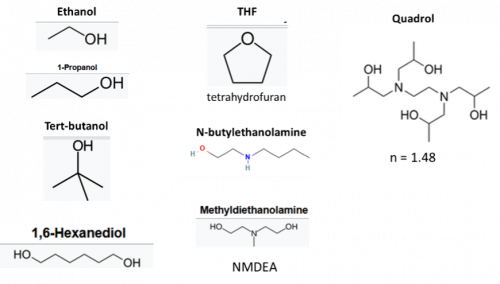
Figure 1: Reagents used in dehydration protocols.
Dehydration
Dehydration is performed over a series of wash steps where the amount of solvent is gradually increased so the sample can ultimately be infused with high n organic solvents. Figure 1 shows commonly used dehydration reagents. Dry methanol has been shown to disrupt fluorescent protein (FP) signals, so most protocols have moved to longer alcohols at low temperatures (4o C), including dry ethanol (Tocris, Visicol), 1-propanol or tert-butanol maintained at basic pH (uDISCO, BABB, PEGASOS, FluoClearBABB) preserve FP signal. When reaching the highest alcohol concentration wash steps, it is important to keep the solutions covered to avoid water absorption from the air- especially in humid environments.
Some protocols combine the dehydration and delipidation steps. The SOLID protocol uses hexanediol with tert-butanol + n-butyldiethanolamine, while tetrahydrofuran (THF) provides dehydration and delipidation simultaneously in Fast3DClear and some DISCO methods.
Delipidation
In general, alcohols alone are poor at solubilizing lipids, (Christie, 1993) so THF or a mixture of dichloromethane/ methanol is used (some DISCO methods). The iSHANEL protocol (Zeng, 2025) was developed for brain tissue that is very lipid dense. Its aggressive delipidation protocol uses several washes of CHAPS buffer with NMDEA to simultaneously decolorize and remove some lipids, followed by dehydration and solvent-delipidation. Samples are rehydrated, permeabilized, retreated with CHAPS/NMDEA, dehydrated and delipidated a second time before index-matching.
Lipid removal is not strictly necessary for clearing (Tocris and Visicol), especially if water is removed and replaced with a high-n material that matches the index of the remaining components. This approach preserves membranes for common diffusion labels used in neuroscience (e.g. DiI).
IHC Labelling
Some protocols (Visikol, iDISCO) perform the IHC and antibody labeling before the dehydration steps. Others (Tocris, iPEGASOS) go through a rehydration process before labeling. Dehydration can alter antibody/epitope binding, so labeling must be validated with each protocol. Tocris and Visikol provide a list of validated antibody labels on their websites.
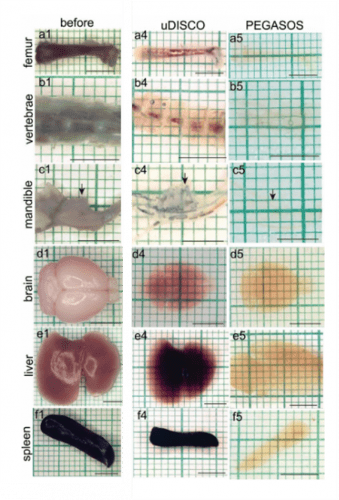
Figure 2: Performance comparison of dehydration clearing methods uDISCO and PEGASOS. Hard and soft tissue samples were harvested from adult C57BL/6 mice (P60) and processed. Arrows in c indicate the mandibular first molar. Scale bars, 5mm. Used without modification under Creative Commons Attribution 4.0 International License from (Jing, 2018) Supplemental Figure 4.
Index-Matching
A number of organic solvents can be used to match the n of the dehydrated sample before imaging (Figure 3). The aromatics, methyl salicylate and xylenes, (Jayakumar, 2024) BABB (solution of BA and BB), DBE, and DPE all have very high n and can be used alone or with other materials. Typically, samples cannot be stored in these solvents for long periods unless antioxidants are added (Molbay, 2021). The n of the solution can be adjusted by adding poly(ethylene glycol) methacrylate (Zhu, 2024). FP fluorescence is preserved by maintaining basic pH with Quadrol (Jing, 2018)) or N-butyldiethanolamine. (Zhu, 2024)
Commercially available kits from Tocris and Visicol offer reversibility and lower toxicity. Tocris Tissue Clearing Pro for tissues and organoids is a proprietary solvent-based clearing solution with an n of 1.48-1.53. Visikol HISTO contains BA, PEG and TCE that enables storage of cleared samples for over 6 months in the dark at room temperature. Cargille Labs also offers a number of oil-based index-matching liquids.
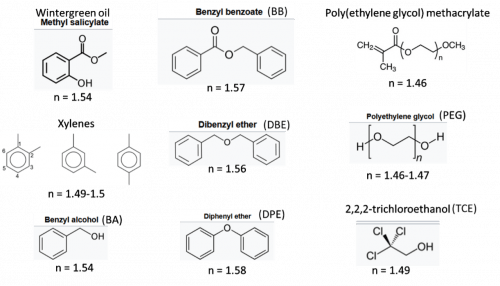
Figure 3: Reagents used in organic index-matching solutions with their abbreviations and refractive indices.
Performance
The various methods described above have been used successfully to image everything from individual neurons in whole brains, to whole embryos and animals, to human tissue biopsies. Figure 4 shows an example of a whole mouse kidney cleared with the FDISCO method imaged with light sheet fluorescence microscopy.
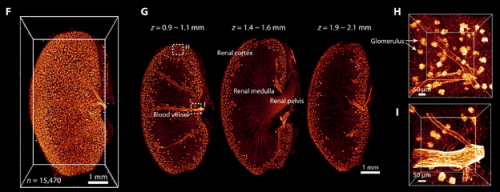
Figure 4. 3D visualization of the vasculature in the kidney after FDISCO clearing. The vasculature was labeled by injection of CD31-A647 antibody. (F) 3D reconstruction of blood vessels and glomeruli in the kidney. (G) Images at gradient depth. The glomeruli were mainly distributed in the renal cortex. (H and I) High-magnification views of the dashed boxed regions in (G). Used without modification under Creative Commons Attribution 4.0 International License from (Qi, 2019) Figure 5.
Generally, researchers select the protocol that has the least number of steps to achieve their research goals. Table 2 summarizes the clearing steps involved in each method with the sorts of signals that can be detected. Delipidation destroys membranes and the ability to label with the membrane-diffusive dyes common in neuroscience (DiI). Samples can also become quite fragile after decalcification and delipidation. Some methods destroy FPs or render them non-fluorescent, although the FP signal can be “boosted” with IHC labelling. Commercial options and the Fast3DClear protocol allow for reversible clearing which allows researchers or clinicians to perform traditional sectioning and processing (e.g. H&E stain) at a later date.
Most dehydration methods suffer from tissue shrinkage that can limit the imaging resolution and the ability of antibody labels to penetrate the tissue. Figure 2 shows up to 40% shrinkage, especially in the lipid-rich brain. (Jing, 2018) It is recommended that researchers start with thinner sections (1-2 mm) and work their way up to whole organs and animals to optimize clearing, permeabilization and IHC labeling conditions. Shrinkage can be used to the researcher’s advantage by reducing the total thickness of the sample, enabling full-volume imaging with a smaller-working distance objective lens.
Organic index-matching solutions are incompatible with polystyrene labware and can damage objective lenses by dissolving optical adhesives and coatings. It is also well known that fluorescence spectra can change in different solvents (Eliat, 2022) necessitating a re-evaluation of filter sets and light sources, especially in multiplexed samples.
Despite many issues, dehydration methods remain popular because they are relatively fast and easy to implement. Next month, we will discuss the hydrophilic and hydrogel-based methods.
For additional reading about the mechanisms behind tissue-clearing see (Yu T. Z., 2021)
Supporting Your Research
As tissue clearing and advanced 3D fluorescence imaging methods continue to expand research possibilities, having the right experimental tools is essential. FluoroFinder supports your work with an integrated Microscopy Spectra Viewer for comparing fluorophores under solvent- and instrument-specific conditions. With access to the most comprehensive fluorophore database and antibody search tools, FluoroFinder helps streamline experiment planning so you can focus on generating clear, reproducible results from your cleared tissue imaging studies.
References:
- Bashkatov, A. G. (2000). doi:10.1117/12.405952.short
- Christie, W. (1993). https://www.aocs.org/resource/preparation-of-lipid-extracts-tissues/#C
- Eliat, F. S. (2022). doi:10.1038/s41598-022-09303-9
- Foster, D. N. (2019). doi:10.1038/s41598-019-50359-x
- Gao, P. R. (2024). doi:10.3389/fncir.2024.1345692
- Jayakumar, D. L. (2024). doi:10.4103/jofs.jofs_64_24
- Jing, D. Z.-P. (2018). doi:10.1038/s41422-018-0049-z
- Kosmidis, S. N. (2021). doi:10.1016/j.crmeth.2021.100090
- Krivosudsky, O. D. (2016). doi:arXiv:1612.06425v1 [physics.bio-ph] 19 Dec 2016
- Molbay, M. K.-L. (2021). doi:10.15252/msb.20209807
- Qi, Y. Y. (2019). doi:10.1126/sciadv.aau8355
- Schwarz, M. S. (2015). doi:10.1371/journal.pone.0124650
- Yu, T. Z. (2021). doi:10.1016/j.isci.2021.102178
- Zeng, F. H. (2025). doi:10.3390/ijms26083569
- Zhu, J. L. (2024). doi:10.1038/s41467-024-52560-7