Two-dimensional (2D) cell cultures represent a cornerstone of scientific research due to their relative simplicity and the establishment of standard techniques over time. In recent years though, 3D cell culture models have risen in popularity for the more physiologically relevant information that they can provide. This article gives a brief overview of both approaches, including their respective advantages, before delving into the main types of 3D cell cultures that have been developed in recent years. We also speak with subject matter experts from Corning Life Sciences and MilliporeSigma (the life science business of Merck KGaA, Darmstadt, Germany in the U.S. and Canada) to learn about some of the innovative products that are available for 3D cell culture applications.
2D Cell Cultures
2D cell culture involves growing cells on a flat surface, such as a culture dish or microplate, where they form a monolayer. Depending on the type of experiment being performed, the cells are then treated with small molecule drugs, cytokines, or other agents, and specific responses are measured with techniques including immunofluorescent microscopy, enzyme-linked immunosorbent assay (ELISA), and western blot. Advantages of 2D cell-based assays include their cost-effectiveness, scalability, and ease to set up and analyze. Yet, because 2D cell-based assays do not accurately replicate physiological conditions, there can often be a disconnect between in vitro and in vivo research.
3D Cell Cultures
During 3D cell culture, cells are grown in a three-dimensional space, typically through the use of specialized cultureware or a scaffold. A major advantage of 3D cell culture is that, by better mimicking cell-cell and cell-extracellular matrix (ECM) interactions compared with 2D methods, it can improve in vitro–in vivo correlations. “3D cell culture also helps to reduce animal testing,” says Hilary Sherman, Senior Applications Scientist at Corning Life Sciences. “With the passing of the FDA Modernization Act 2.0 in 2022, there is interest in finding in vitro models that can serve as alternatives to using live animals for research.”
Joseph Boyd, Ph.D., Research Scientist in the Cell Biology group at MilliporeSigma, notes that another important advantage of 3D cell culture is its applicability for investigating phenomena such as vascularization, barrier integrity, and drug absorption. “In addition, certain types of 3D cell cultures, such as patient-derived organoids, can be used in drug screening and toxicity assessments to predict side effects and reveal patient-to-patient variability,” he says. “Such data can be extremely valuable for making decisions on proceeding with a therapeutic project.”
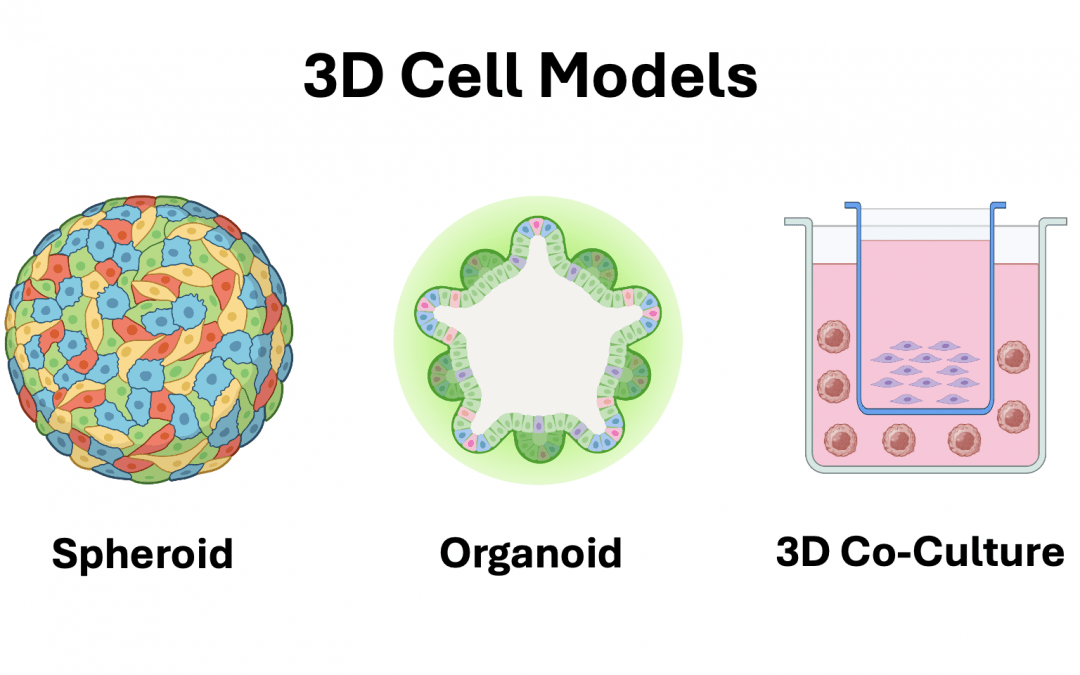
Types of 3D Cell Culture
3D cell cultures vary in terms of complexity and the types of applications they support.
Spheroids
One of the most commonly used models, spheroids are formed by the aggregation of one or more cell types, either in low-attachment cultureware, via suspension in a biomatrix, or using the hanging-drop method (1). They are especially popular for studying the tumor microenvironment, where they allow for investigating cell-cell signaling, evaluating nutrient and oxygen gradients, and assessing the effects of treatment with small molecule drugs. In an oncology setting, spheroids are usually generated from established tumor cell lines or primary tumor cells, while other fields of research use cell types including primary human hepatocytes, choroid-retinal vascular endothelial cells, human kidney epithelial cells, and others as the source material.
Organoids
Another well-regarded 3D cell culture model, organoids are derived from embryonic stem cells, induced pluripotent stem cells, or adult stem cells that have been harvested from primary tissues. They generally require a 3D scaffold consisting of ECM proteins to facilitate their growth and are formed by introducing carefully curated mixtures of cytokines, growth factors, and other biomolecules into the culture medium to promote cellular differentiation into miniaturized organs. “Because most organoids comprise multiple cell types, they more closely resemble the in vivo architecture and physiology than spheroids,” says Boyd. Organoids have been used to investigate normal growth and development, and for studying conditions including Alzheimer’s disease, diabetes, and cancer.
Microphysiological Systems
By using microscale channels to regulate cell microenvironments – including shear stress, fluid flow, and gradients of nutrients, oxygen, and signaling molecules – microphysiological systems enable researchers to study organotypic behaviors in a controlled and reproducible manner. Within this category, organ-on-a-chip and body-on-a-chip systems have garnered the most attention. Organ-on-a-chip systems allow for modeling cellular interactions within specific organs and have been developed for organ types including the lung, pancreas, and heart, as well as the blood-brain barrier2. Body-on-a-chip systems, first reported in 2004, are multi-organ platforms that are often designed to emulate the human physiological response to a drug, capturing both drug efficacy and potential toxicities in other organs3,4.
Addressing Imaging Challenges With Tissue Clearing
3D cell cultures are more difficult to image than 2D cell cultures due to their thickness and opacity. A common workaround involves sectioning 3D cell cultures prior to antibody-based staining, but this is both laborious and time-intensive, as well as risks causing tissue deformation. Alternatively, researchers can use a tissue clearing agent to allow for section-less imaging. Commercial products include Visikol® HISTO-M™, which is available from MilliporeSigma, and Corning® 3D clear tissue clearing reagent. Benefits of these products are that they are compatible with fluorescent proteins and other fluorescent labels, and can be used with microplates and automation.
Products for 3D Cell Culture
As the use of 3D cell culture has grown, so has the range of products to support these types of studies. “Over the past few decades, Corning has pioneered a broad array of tools that provide easier access to in vivo-like 3D cell models,” reports Sherman. “These include Corning Matrigel® matrix, which is one of the most widely used models for 3D cell culture, and Transwell® permeable supports, which have utility for applications including co-culture, working with polarized cells, and studying the air-liquid interface. We have also developed the Corning Matribot® bioprinter, the first benchtop bioprinter designed to handle Corning Matrigel matrix using a cooling syringe printhead technology that lets you dispense 3D droplets or droplet arrays for organoid applications.”
MilliporeSigma’s product offerings include Millicell® Microwell 96-well plates, which use an array of microwells to promote homogeneous single organoid formation. “The spheroids and organoids generated in Millicell Microwell plates form in a single focal plane, which standardizes high throughput imaging applications,” explains Boyd. “Importantly, to avoid disturbing the cultures, each plate features an independent pipetting port for safe media exchange and liquid handling.” MilliporeSigma has also recently expanded its 3dGRO® portfolio with colorectal cancer and pancreatic ductal adenocarcinoma cancer (PDAC) biobanks. “Each of these new biobanks comprises 20 lines from individual patients, representing a range of genetic backgrounds, driver gene mutations, tumor sites and cancer stages,” says Boyd. “By conserving the original genetic and phenotypic characteristics of the primary tissue, 3dGRO® cancer organoids can help researchers to determine the underlying mechanisms of disease and predict responses to chemotherapeutics.”
Supporting Your Research
Whether you’re working with 2D or 3D cell cultures, FluoroFinder has the tools you need to help streamline your experimental design. Use our Antibody Search tool to quickly find antibodies for your chosen target, species, and application. And, for microscopy-based research, use our Spectra Viewer to identify the right fluorophores for your microscope’s laser and filter configurations.